AJAA Index | Virtual Library | Magazine Rack | Search
Abstract. Biological control is defined broadly as the "use of natural or modified organisms, genes, or gene products " to reduce the effects of pests and diseases. Physical control is the use of tillage, open-field burning, heat-treatment (pasteurization), and other physical methods, usually to eliminate pests or separate them from the crop. Chemical control is the use of synthetic chemical pesticides to eliminate pests or reduce their effects The many approaches to biological control can be categorized conventionally into 1) regulation of the pest population (the classical approach), 2) exclusionary systems of protection (a living barrier of microorganisms on the plant or animal that deters infection or pest attack), and 3) systems of self-defense (resistance and immunization). The agents of biological control include the pest- or disease-agent itself (sterile males or avirulent strains of pathogens), antagonists or natural enemies, or the plant or animal managed or manipulated (immunized) to defend itself. The methods range from 1) conserving and making maximum use of indigenous (resident) biological control through cultural practices, 2) making one-time or occasional introductions of genes or natural enemies that are more or less self-sustaining and 3) making repeated introductions of a biocontrol agent (e.g a microbial pesticide). Biological, physical, and chemical treatments and pest controls can be integrated into holistic plant-health care also known as integrated crop and pest management. Eight principles of plant health care are offered: 1) know the production limits of the agroecosystem; 2) rotate the crops; 3) maintain soil organic master; 4) use clean planting material; 5) plant well-adapted, pest-resistant cultivars; 6) minimize environmental and nutritional stresses; 7) maximize the effects of beneficial organisms; and 8) protect with pesticides as necessary.
Key words: plant diseases, fungi, bacteria, viruses, nematodes, plant breeding, cultural practices, tillage, planting date, non-pathogens, biocontrol agents
In considering the contributions of biological pest control to a sustainable agriculture, it may be useful first to examine briefly some of the advantages and disadvantages of each of the major methods by which pests can be controlled. The major methods of pest control can be grouped into three categories of 1) physical control, 2) chemical control, and 3) biological control. These broad categories, in turn, can be combined into integrated pest management (IPM), integrated crop and pest management (ICPM), or, as will be used in this article, holistic plant-health care or simply plant-health caret The equivalent for livestock is integrated livestock management or animal health care.
Physical control includes tillage to control weeds, open-field burning to control pests (Hardison, 1976), solar heating the soil beneath clear plastic tarp (Katan, 1981), elimination of pathogens from milk or rooting media by mild heat-treatment (Baker, 1962), the production of pathogen-free plants from tissue culture started with clean meristem or shoot tips (Holings, 1965), and the physical separation of crop from a potential pest attack by choice of planting date. The production of pathogen-free plants from tissue culture is nonpolluting and, along with indexing of seeds, is the best or only acceptable method to eliminate some viral and bacterial pathogens so that the planting material can be certified as pathogen-free (Holings, 1965). On the other hand, tillage is energy-expensive (Phillips et al., 1980) and contributes to soil erosion; the trend in the United States is therefore toward less tillage to conserve energy, reduce soil erosion, and make U. S. agriculture more sustainable. Open-field burning contributes to air pollution and may, over the long term, have a negative effect on the organic master content of soil; the tendency is therefore toward reduced use of burning, and legislation has been introduced in some states to regulate or even ban open-field burning. Solar-heating the soil involves the capture of incoming solar radiation beneath clear plastic sheeting placed on the soil surface. It is a safe method by which plant-parasitic nematodes, soilborne fungal pathogens, soil-inhabiting insect pests, and some weed seeds can be eliminated by heat treatment of soil in gardens, vegetable fields, and orchards (Ashworth et al., 1982), but is usually not economical except for high-value crops and in areas of abundant sunshine.
Chemical control is used in this report to mean control of pests with chemical pesticides. The problems of chemical pesticides have been reviewed amply and need not be restated here. While some pesticides must be abandoned because of their unacceptable nontarget effect, there will always be a need in agriculture for safe and selective chemicals to limit the effects of pests. More significantly, it is becoming increasingly more difficult and expensive to find new kinds of synthetic chemical pesticides. The chemical pesticide industry has therefore been described as a "maturing industry."
Biological control is the control of one organism by another (Beirner, 1967). This control may be expressed as either a longer population of the pest (DeBach, 1964) or as a restriction or prevention of the severity or incidence of pest damage without regard to the pest population (Cook and Baker, 1983). Biological control depends on knowledge of biological interactions at the ecosystem, organismal, cellular, and molecular levers and often is more complicated to manage compared with physical and chemical methods. Biological control is also likely to be less spectacular than most physical or chemical controls but is usually also more stable and longer lasting (Baker and Cook, 1974). In spite of biological controls having been used in agriculture for centuries, as an industry biological control is still in its infancy.
Biological control is now being considered for an increasing number of crops and managed ecosystems as the primary method of pest control. One reason for its growing popularity is its record of safety during the past 100 years considered as the era of modem biological control (Waage and Greathead, 1988). No microorganism or beneficial insect deliberately introduced or manipulated for biological control purposes has, itself, become a pest so far as can be determined, and there is no evidence so far of measurable or even negligible negative effects of biocontrol agents on the environment (in Cook, Chairman; 1987). Another reason for considering biological control over other methods is untapped potential; biological control is underused, under exploited, underestimated and often untried and therefore unproven. The new tools of recombinant DNA technology, mathematical modeling, and computer technology combined with a continuation of the more classical approaches such as importation and release of naturel enemies and improved germplasm, breeding, and field testing should quickly move biocontrol research and technology into a new era.
Biological control describes the normal state of affairs in naturel undisturbed ecosystems, where populations of organisms exist in a dynamic equilibrium and species or individuals unable to compete or to find an ecological niche are replaced by those that can. With sufficient knowledge, it becomes possible to manipulate this equilibrium so as to favor some organisms more than others. Th us be gins agriculture , silviculture , gardening, and other similar activities that favor a few desirable plant or animal species, or subsets of species (cultivars, breeds, strains), that otherwise could not succeed and might even become extinct.
This report is focused on biological control of pests and diseases of plants important in farmland, orchards, and other agroecosystems. Many of the examples discussed involve the control of diseases of wheat (Cook, 1986c), but the concepts presented are just as applicable to pest and disease control on other crops and in other managed ecosystems, including urban and recreational areas.
This report also introduces some principles of holistic plant-health care, which involves extensive use of biological control integrated with physical and chemical treatments and pest controls as appropriate and compatible with the goals of making agriculture more sustainable.
Biological control was discovered by trial and error and then practiced in agriculture long before the term itself came into use (Baker and Cook, 1974). One example is the ancient practice of not growing the same crop species in the same field more frequently than every second or third year or even longer. Such crop rotation allows time for the pest or pathogen population in soil to decrease below some economic threshold because of the predatory, competitive, and other antagonistic effects imposed by the associated microflora and fauna. In other words, crop rotation allows time for the naturel soil microbiota to sanitize the soil, especially with regard to the more specialized plant parasites and insect pests that are highly dependent on their host crop to maintain their populations.
The era of modern biological control, involving the deliberate transfer and introduction of naturel enemies of insect pests, was launched 100 years ego with the highly successful introduction of the vadalia beetle from Australia to California in 1888 to control the cottony cushion scale of citrus. In 1914, the German plant pathologist C. F. von Tubuef wrote a somewhat speculative article entitled "Biologische Bekampfung von Pilzkrankheiten der Pflanzen." This is apparently the first reference in the scientific literature to the term "Biologische Bekampfung" or "biological control" (Baker, 1987). In 1916, L. O. Howard referred to control of the cottony-cushion scale insect by the vadalia beetle as a "biological method" and in 1919, H. S. Smith called it biological control (Baker and Cook, 1974).
About 80 years ago, a gene for resistance in wheat to wheat stem rust caused by Puccinia graminis f. sp. tritici was successfully transferred for the first time by crossing a rust-resistant with a rust-susceptible wheat plant (Biffen, 1906). Thus began the practice of introducing genes for resistance to pests, first by conventional methods and now expanded to include genetic engineering by use of recombinant DNA technology. Lupton (1984) gave emphasis to this approach in his presidential address to the Association of Applied Biologists in Great Britain, entitled, "Biological Control: The Plant Breeder's Objective." Moreover, the boundaries that once existed between these two approaches to biological control--transfer of whole organisms and transfer of genes--are beginning to disappear because of the tools of recombinant DNA technology. For example, a gene for production of an endotoxin by a strain of Bacillus thuringiensis, lethal to certain insect pests of crops, has now been transferred by recombinant DNA technology to tobacco and tomato and shown to function in these plants as genes for resistance to the toxin-sensitive insect pests of these plants (Vaeck et al., 1987).
It is commonly argued that biological control as a concept should exclude host plant resistance to pests and diseases achieved by introduction of genes through plant breeding (R. Baker, 1985). Such a definition puts this science into the awkward position that the use of a plant pathogen with certain genes for virulence to maintain a population of susceptible weed plants at or below some economic threshold would qualify as biological control, but the converse, the maintenance of a pathogen population at or below some economic threshold by deployment of certain genes for resistance in the crop plants, would not qualify as biological control. As another incongruity, the bt gene for production of the insect toxin expressed in the insect pathogen Bacillus thuringiensis would qualify as biological control but the same gene expressed in plants, as a gene for resistance to insect pests, would not qualify as an example of biological control. Such a narrow definition is artificial and scientifically indefensible. Perhaps Lupton (1984) has stated it best: "accelerating or diverting evolutionary processes in order to obtain genotypes adapted to [man's] needs are a most important example of the application of biological control to agricultural and horticultural crops."
DeBach (1964) defined biological control as "the action of parasites, predators, or pathogens in maintaining another organism's population density at a longer average than would occur in their absence." This definition covers some highly successful biological controls of insect pests with naturel enemies, but it does not accommodate some other highly successful controls accepted in other disciplines as examples of biological control. For example, citrus tristeza virus is controlled in Brazil by inoculating the citrus trees with a mild virus, which then protects the trees against the more severe strains (Costa and Muller, 1980). "Cross protection" was first shown by H. H. McKinney in 1929 to have potential for biological control of plant viruses. Plant pathologists refer to cross protection for control of plant viruses as biological control.
The many biological controls that fall outside the narrow definition have been variously labeled as "biological methods of control," "biological forms of control," and "biological pest suppression" (Jones and Solomon, 1974; Lundholm and Stackerud, 1980). All of these terms, like H. O. Howard's original "biological method," mean simply "biological control."
I propose that scientists adopt a definition of biological control that can encompass what scientists worldwide have long called biological control. Such a broad definition is given in the recent "Report of the Research Briefing Panel on Biological Control in Managed Ecosystems" (in Cook, Chairman; 1987) as: "the use of naturel or modified organisms, genes, or gene products to reduce the effects of undesirable organisms (pests), and to favor desirable organisms such as crops, trees, animals, and beneficial insects and microorganisms." A major achievement of this report is that it provides a scientific framework that accommodates all approaches to biological control for all categories of pest agents. This scientific framework breaks clown into strategies, agents, and methods discussed below.
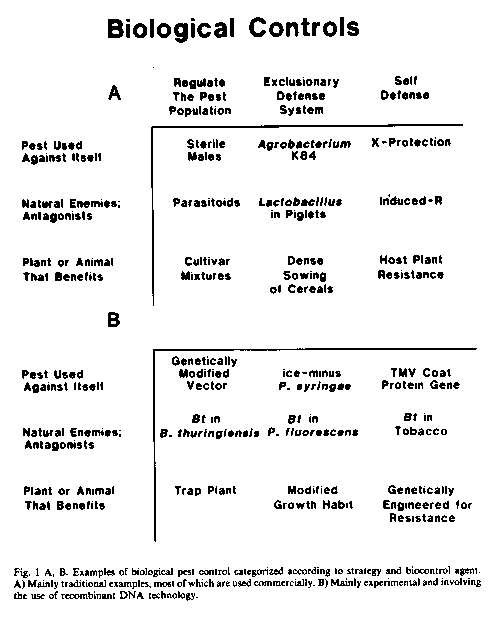
The seemingly unlimited array of different approaches to biological control can be categorized quite simply into three strategies (in Cook, Chairman; 1987): 1) Regulation of the pest population; 2) Exclusionary systems of protection; and 3) Systems of self-defense (Figure 1).

This approach to biological control uses biological control agents to regulate the pest population at or below an acceptable threshold of pest population and/or plant injury. Some 10-15 weed species are now controlled partly or completely by insects or plant pathogens released because of their ability to feed on or parasitize these weeds. The population of soilborne plant pathogens can be regulated biologically by taking advantage of resident antagonists through a cultural practice such as crop rotation. Regulation of the pest population is the only acceptable strategy for many if not most insect pests, plant parasitic nematodes, rodents, and weeds.
This strategy aims at the use of beneficial rnicroorganisms as a living barrier to exclude infection or deter pest attack. The control of crown gall of ornamental shrubs (e.g. roses) and certain fruit trees is an example. Bare-root transplants are dipped into a suspension of cells of the avirulent antibiotic-producing strain K84 of Agrobacterium radiobacter, which then protects the roots against infection by the virulent antibiotic-sensitive A. tumefaciens (Kerr, 1980). An example with livestock is the use of a nonpathogenic Lactobacillus fermentum introduced into newborn piglets to exclude the pathogenic Escherichia coli from the intestinal lining and thereby protect the piglets from neonatal scours (Hill, 1985). This approach also has potential for post-harvest protection of fruits against rots caused by fungi. These fungi infect through wounds or abrasions in the skin of the fruit and traditionally have been controlled by submerging the fruit in a fungicide solution after harvest and before packing. Several such rots have now been controlled experimentally by use of nonpathogenic antifungal bacteria instead of fungicides (Wilson and Pusey, 1985).
This strategy accepts that the pest population cannot be regulated directly and that some amount of infection or pest attack will occur. However, the opportunity to use biological control still exists, namely, through the systems of self-defense in the plant itself that can limit disease severity or pest damage. These mechanisms of self-defense may be constitutive, i.e. they may be an "up and running" feature of the living plant, they may occur as a response to attempted infection or pest attack, or they may be dependent for expression on vigor and health (vitality) of the plant. Avoiding the stresses that predispose plants to disease is critical to maximizing the kinds of self defense dependent for expression on vigor of the suscept, especially defense against the so-called opportunistic pathogens and weak parasites (discussed in more detail below).
Resistance to more specialized disease-agents and pests traditionally has been obtained in plants by genetic improvements, and in animals by immunization. Some plants can also be "immunized" (this term is used advisedly because the mechanism of protection in plants is very different from that in animals) against their pathogens by prior inoculation with an avirulent strain of the pathogen, or in some cases by inoculation with an organism pathogenic on another plant (Kuc, 1982). Often, these agents are pathogens of other crops and therefore are not used commercially for biological control. Nevertheless, considerable "induced resistance" or "immunization" of plants to diseases probably occurs naturally in response to the many kinds of microorganisms that make contact with plants below- or above-ground during the growing season.
The agents of biological control can include 1) the pest or disease-agent used against itself, 2) antagonists or natural enemies of the pest or disease-agent, or 3) the host plant or animal managed or manipulated to defend itself (Figure 1).
Examples of an insect pest used against itself include the release of sterile males or the use of behavior-modifying pheromones produced by insects to regulate the population (strategy 1) of certain insect pests over wide geographic areas or within specific orchards or other agroecosystems. A disease-agent can also be used against itself in an exclusionary system of defenses (strategy 2), such as the use of an ice-minus (ice-) strain of Pseudomonas syringae, applied to the foliage of frost-sensitive plants, to exclude ice-nucleation-active (INA) strains of P. syringae (Lindow, 1983). A nonpathogenic ice strain can be produced from a wild-type INA strain by a single deletion-mutation using the tools of recombinant DNA technology Such strains were recently approved for field testing in California. The example of cross protection with a mild strain of a plant virus for biological control of severe strains of the virus illustrates the pathogen used to initiate self-defense (strategy 3) within the otherwise full, susceptible (sensitive) plant. It is now, possible to obtain significant control of tobacco mosaic virus (TMV) in tobacco and tomato by incorporation of the TMV gene for coat-protein production into the plant genome (Abel et al., 1986) such transgenic plants apparently are better equipped than their progenitor to defend themselves against TMV. This approach avoids the risks sometimes associated with deliberate inoculation of plants with mild strains of plant viruses (Fulton, 1986).

The antagonists and naturel enemies of pests include the classical biocontrol agents imported, mass-reared, and released to control a pest population (strategy 1). This approach, used against insect pests and weeds, is one of the major ongoing success stories in biological control in the world today. Other success stories include the use of antagonists applied to pruning wounds to provide an exclusionary system of protection (strategy 2) against pathogens (Corke, 1978; Rishbeth, 1979). One emerging technology is the use of plant associated microorganisms such as rootcolonizing bacteria (rhizobacteria) to protect germinating seeds and roots against soilborne pathogens (Cook et al., 1987a; Schroth and Hancock, 1982). The rhizobacteria can be introduced on the seeds, seedpieces, or cuttings and are selected for their ability both to colonize the germinating seeds and roots and to inhibit the target pathogens (Cook and Baker, 1983). As a third approach, the ability of plants to defend themselves (strategy 3) can sometimes be enhanced or the time required for a resistance-type response in the plant can be shortened by prior inoculation of the plant with an agent totally unrelated to the pathogen (Kuc, 1982; see previous section).
Plants can also be used as agents of biological control in any one of the three strategies outlined above. The plant can be used as a trap to regulate the population of plant parasitic nematodes (strategy 1). An example is the growing of Crotalaria spectabilis as a cover crop in peach orchards to reduce the population of root-knot nematodes (McBeth and Taylor, 1944). The female larvae responsible for root-knots in fully-susceptible host plants were shown to penetrate the roots of C. spectabilis, but because giant cells essential to the parasitic relationship did not form, the immobilized females did not lay eggs. An example of an exclusionary system of defense (strategy 2), using the plant, is to increase the seeding rate or narrow the row spacing of the crop so as to exclude weeds. The various narrow rowspacings selected for small grains, for example, are mainly for weed control by exploiting the competitive ability of the crop. Finally, plants can be managed culturally or manipulated genetically so as to maximize their physiological and biochemical systems of self defense (strategy 3). This is accomplished by use of practices to minimize predisposing stresses that limit ability of the plant to defend itself, or by conventional or nonconventional breeding designed to improve the ability of the plant to defend itself.
The methods of biological control can be categorized into a logical order of 1) conserving or making maximum use of the indigenous biological controls, 2) making one-time or occasional introductions of genes or organisms that then become somewhat self maintaining, and 3) making repeated introductions of biocontrol agents in the form of products such as microbial pesticides.
Any program aimed at biological control of a specific pest or disease should begin by making maximal use of the indigenous or constitutive (background) biological controls. This may be accomplished by use of cultural practices intended to take maximal advantage of or at least not upset the beneficial biological interactions that limit the population or damaging effects of pests and diseases. Crop rotation is one such practice. Tillage is another such practice; besides providing physical control of weeds, tillage can also 1) intensify the naturel biological stresses on populations of pathogens in soil, 2) accelerate the displacement of pathogens by nonpathogens in crop residue in soil, and 3) relieve predisposing stresses on the crop such as may occur m response to compact soil, poor drainage, or a pressure pan (Allmaras et al., 1988). The means to maximize or at least conserve the biological controls provided by the soil microbiota, indigenous beneficial insects, and existing resistance in plants to pests and diseases can and have been discovered mostly by trial and error but could be used with much greater success if more fundamental knowledge of these systems and of the biological interactions were available.
To the extent that the indigenous biological control is inadequate or no longer effective, one time or occasional introductions of naturel or modified organisms or genes generally are considered. Many pests remain for years in a state of suppression, until some change in the farming practice upsets the balance and permits the pest to flourish. In other cases, a pest may escape its naturel enemies, as when inadvertently introduced into a new environment. The intent of a one-time or occasional introduction is to reestablish a favorable balance between the crop plant and its pest, or between naturel enemies and the pest, and therefore restore some semblance of the original balance or create a new state of pest suppression. Wheatstem rust is managed in the Great Plains and Prairie Provinces of North America by strategic deployment and pyramiding of genes for resistance in the network of cultivars grown in this large area in response to the genes for virulence identified and monitored in the mixture of races of the pathogen active in the area (Roelfs, 1988). The classical approach of introducing exotic naturel enemies of naturalized arthropod pests and weeds mentioned above also exemplifies this method.
As a third general method, biological control agents can be introduced repeatedly, perhaps with each sowing of the crop or perhaps several times during the growing of a crop. Herein lies the opportunity for insect pathogens such as Bacillus thuringiensis or baculoviruses for insect control (Payne, 1988), the use of specialized fungal pathogens of weeds such as mycoherbicides (Templeton et al., 1986), and the application of antagonists of plant pathogens to seed or other planting material (Cook and Baker, 1983) for disease control. Microbial "pesticides" offer several advantages over synthetic chemical pesticides, including no known or only limited negative effects on the environment and no known problems thus far of pests becoming resistant to the microbial agent(s). Some antibiotics, if applied in purified form as a chemical, may actually be more hazardous in the environment than some synthetic chemical pesticides. These hazards are greatly reduced when a "live delivery system" is used; the chemical is more likely to be released slowly, exactly where needed, and at much longer concentrations than if purified and applied as single or multiple doses. Microbial biocontrol agents also tend to fill rather than create vacant biological niches and usually affect only the target pest species, and many successful antagonists use more than one mechanism to inhibit sensitive pathogens. Microbial biocontrol agents are therefore less likely than chemical pesticides to provide an opportunity for a new pest or resurgence of the original pest.
Holistic health has become somewhat popularized with regard to humans and animals, but is rarely considered in relation to plants. Below is an attempt to summarize some of the scientific principles that should be taken in account as approaches to holistic plant-health care (Figure 2). Most of these principles are consistent with the approaches to integrated crop and pest management (ICPM).
Production limits of the agroecosystem might also be called the law of the most limiting factor in the environment. The limiting factor in the environment to yield of wheat in most areas of the world is available water. The limiting environmental factor to yield of corn could be water, growing degree-days, or possibly carbon dioxide available for photosynthesis, depending on the area. For yield of potatoes, the limiting environmental factor is thought to be total sunshine during the period of vegetative growth (van der Zaag, 1984). Crop yields can also be limited by depth of soil and soil fertility.
The management system imposed on a crop in any given year or area should take into account the most likely yield limiting factor in the environment for that crop, year, and area. A crop managed through choice of planting date or fertilized for a yield higher than is attainable based on the most limiting environmental factor may then be subject to other stresses and thereby become more vulnerable to damage by the weak and facultative type parasites. The control in eastern Washington State of Fusarium foot rot of wheat, caused by Fusarium culmorum, exemplifies a biological control achieved by knowing the production limits of an agroecosystem (Cook, 1980). The agroecosystem in this case is a wheat-fallow rotation in the low-rainfall (20-40 cm annual precipitation) areas of the state. This disease became important in the early 1960's shortly after farmers in the area began to grow the semi-dwarf wheats heavily fertilized with nitrogen. The disease was 'shown to be favored by the low, midday plant water potentials associated with high rates of N and the resultant large leaf area available for movement of water into the atmosphere through transpiration (Papendick and Cook, 1974). Put quite simply, growers were fertilizing for a yield higher than could be produced or expected with available water. The disease was controlled by reducing the N to rates more in line with the yield potential for the field.
Knowing the production-limits of the agroecosystem is also necessary as a means to recognize and focus on problems when yields are below the attainable. Yields lower than attainable may be sought deliberately by fertilizing or irrigating for the maximum economical yield rather than the maximum yield. In many cases, however, the actual yield is some fraction of the attainable or potential as limited by the environment and paid for as inputs. This situation calls for an accurate diagnosis and usually reveals the action of one or more pests or diseases at work in the field.
Growth, phenology, and productivity models are becoming available for most major crop plants that are driven by thermal-time (Rickman et al., 1985), accumulated days of sunshine (van der Zaag, 1984), available water, and presumably other limiting factors of environment if critical. More research is needed to integrate pest cycles and disease development into these kinds of models (Rouse, 1988) and also to predict more accurately the cycles and risks o climate and weather likely to set the production limits of the agroecosystem.
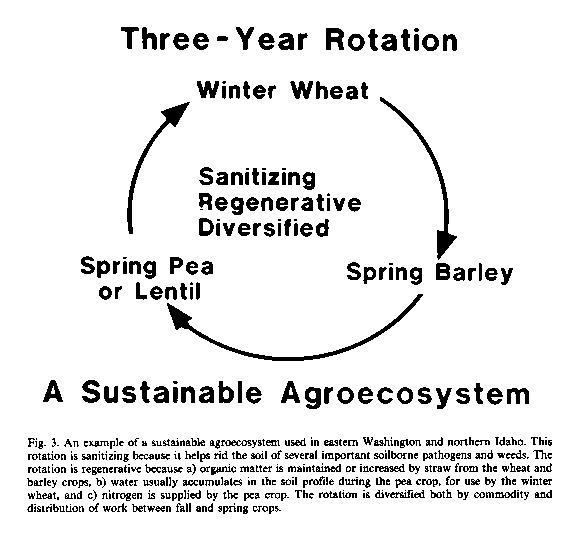
Yields tend to decline with crop monoculture or eventually they stabilize a some level significantly less than attain able if the crop is grown no more that every third or fourth year in the same field. This decline results from the gradual increase in populations of plant parasitic nematodes, root-infecting fungi, and other soil-inhabiting pests and pathogens favored by that crop (Cook, 1984). Simply rotating between unrelated crop species may disrupt the cycles of these organisms. Left without access to the roots of their host, and being unable to compete for the organic substrates in soil or to attack the roots of other crops, these pests succumb quickly to the competitive effects and other stresses imposed by the associated soil microbiota active in the soil.
The growing of winter wheat in the same field in eastern Washington no more than once in three years was nearly as effective as soil fumigation with methyl bromide, chloropicrin, or 1,3dichloropropene + chloropicrin as a means to rid the soil of the most important root pathogens (Cook et al., 1987b). One particular 3-year rotation in eastern Washington uses winter wheat followed by spring barley, spring pea (or lentil), and then back to winter wheat (Figure 3). Two successive crops of spring grains (e.g. barley or wheat) control Cephalosporium stripe caused by C. gramineum in winter wheat, because this disease only occurs on winter wheat or barley. However, any two consecutive crops of wheat or barley (spring- or winter-type) favor take-all caused by Gaeumannomyces graminis var. tritici. A single year to a crop other than wheat, barley, or a susceptible grass is therefore needed to control this disease, which is accomplished by preceding the winter wheat crop with a pea or lentil crop. One grower reported that "he puts more wheat in the bin" now, by cropping only one-third of his ranch to winter wheat each year (3-year rotation), than when he planted half his ranch to wheat each year (2-year rotation) (don Whitman, persona! communication).
Some plant species have special value as rotation crops because of their active role in the sanitization of soils. The example mentioned above of Crotalaria spectabilis used as a trap crop to reduce the population of root knot nematode fits this category. The wheat cultivar Festiquay has shown potential in Australia as a possible trap crop for the cereal cyst nematode Heterodera avenae (Rovira and Simon, 1982); a field can be safely planted to a nematode-susceptible cultivar of wheat after growing Festiquay for two or three years. Riggs et al. (1980) have shown for the soybean cyst nematode H. glycines that rotating susceptible with resistant cultivars of soybeans can reduce the selection pressure for new races of this nematode and extend the useful life of resistant cultivars.
Some root diseases have been shown to increase in a predictable way during the early years of monoculture of the crops but then decline unexpectedly while yields recover as the monoculture is continued. Examples include common scab of potatoes caused by Streptomyces scabies (Weinhold et al., 1964), root rot of sugar beet in Japan caused by Rhizoctonia solani (Hyakumachi and Ui, 1982), Rhizoctonia camping-off of radish (Chef and Baker, 1981), and take-all of wheat caused by Gaeumannomyces graminis var. tritici (Shipton, 1977). All are either shown or thought to be examples of naturel biological control by resident (indigenous) antagonists favored by the presence of the host plant, diseased roots, and/or the particular root pathogen (Cook and Baker, 1983).
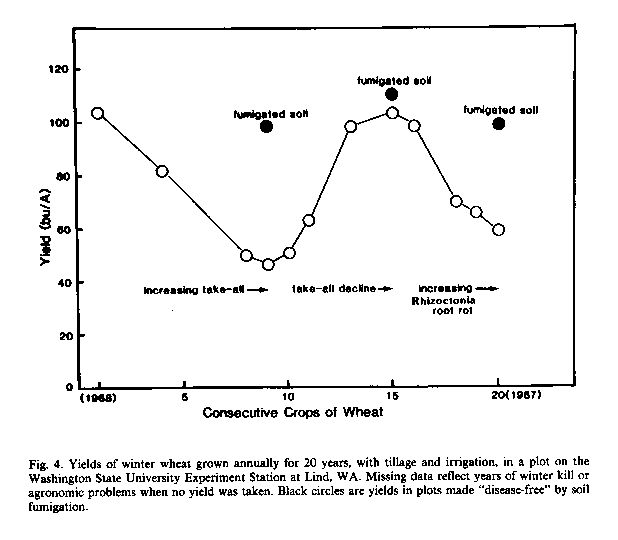
In the Pacific Northwest, the soil microorganisms responsible for take-all decline have been shown to provide highly effective biological control of G. graminis var. tritici in some wheat fields (Cook and Rovira, 1976; Cook and Weller, 1987). Unfortunately, the microbiological factor responsible for take all decline is specific for take-all (Gerlagh, 1968; Cook and Rovira, 1976). It was therefore not surprising, in a longterm experiment in central Washington, that as take-all declined to a near-negligible lever by the 15th year of wheat monoculture, Rhizoctonia root rot began to increase (Figure 4). Possibly another 5-10 years will be required to generate a decline of Rhizoctonia root rot, only to open the way for a third root disease. While crop rotations present the most sustainable method to control soil inhabiting plant pathogens and pests, much can be learned about the potential for biological control of root diseases by studying monoculture-related disease decline phenomena and other examples of pathogen-suppressive soils (Cook and Weller, 1987). Wheat rhizosphere soil taken from fields where take-all declined, for example, has yielded some interesting strains of root-colonizing bacteria (fluorescent Pseudomonas spp.) that now show potential for biological control of take-all when applied as living cells on the wheat seed (Weller and Cook, 1983; see below).
The loss of organic matter content through years of tillage is well known to also cause a loss of soil structure. Poorly structured soils, in turn, tend to compact excessively and to drain more slowly than well-structured soils (Allmaras et al., 1988). Gas exchange and especially the availability of oxygen to roots is also restricted in such soils, which may then limit root growth directly (Russell, 1975) or predispose roots to more severe attack by root pathogens (Stolzy et al., 1965; Allmaras et al., 1988). Even a short period of restricted gas exchange in a poorly drained soil can greatly accelerate Fusarium root rot of bean and pea, apparently because the roots of these crops become physiologically much more susceptible to the Fusarium when denied oxygen (Miller and Burke, 1975; Smucker and Erickson, 1987). Maintaining soil organic matter content so as to maintain soil aggregates and hence good soil drainage and gas exchange in the root zone is probably the single most important factor to achieving and maintaining good root health for crops, and especially to physically limiting the effects of some soilborne plant pathogens that do not respond to biological control by crop rotation because of their broad host range.
Maintaining or elevating the soil organic matter content can also stimulate biological control of soilborne pathogens. It has long been recognized that organic mulches or amendments to the soil can lead to suppression if not out- right elimination (through germination lysis) of the resting propagules of man, root-infecting fungi in the soil (Papavizas and Lumsden, 1980). The exact components and agents of these biological controls are still poorly understood, but involve soil microorganisms stimulated by the amendments.
An example of biological control b. maintaining soil organic matter is the use in the Mt. Tamborine rainforest in Queensland, Australia, of abundant organic amendments to control Phytophthora root rot of avocado caused b. P. cinnamomi (Broadbent and Baker 1975). The goal of this highly successful method is to maintain the soil organic matter content at about 12 percent, com parable to that in the surrounding un disturbed rainforest where P. cinnamomi is native and where many of the native plants are susceptible yet root rot cause by this fungus is relatively rare. The biological control achieved by this practice occurs through complex microbiological interactions between the pathogen an resident antagonists. However, even microbiological suppressiveness of the soils can fail if the soils become water logged during periods of unusual heavy rainfall (Cook and Baker, 1983 )
Only a few of the many potential pest agents of the important annual and perennial crops are controlled by resistant cultivars. However, these few pest agents controlled are also potential] among the most devastating and the least amenable to control by crop rotations or soil treatments, such as the rust and powdery mildew of small grains an southern corn leaf blight of corn--destructive diseases that can quickly increase to epidemic proportions.
An even greater number of chronic subclinical constraints to plant health can be controlled by choosing cultivar best-adapted to the area. This can I accomplished by selecting from among the choice of cultivars developed or, least evaluated locally. Wheat cultivar with ability to avoid or tolerate plant water stress under the semi-arid condition of east-central Washington State tend also to be the most resistant to the Fusarium foot rot (Cook, 1973). Well adapted wheat cultivars are less likely to use up limited soil-water supplies before producing grain; they are also less likely to be injured by local winter conditions (if a winter-wheat cultivar) or to break dormancy before the lest danger of frost has passed in the spring. Such cultivars tend also to be maximally resistant to weak parasites and opportunistic pathogens that depend on predisposition of the plant to cause disease (Cook and Baker, 1983; see below).
Many pest-agents, including disease agents and weed seeds, are introduced into the field or orchard on or with the planting material, or through planting operations. Potato viruses carried in the potato tubers can now be eliminated by regeneration of plants from virus-free tissue cultured from meristematic tissue (Slack, 1980). This approach is also used to produce pathogen-free banana plantlets used to establish new banana plantings in Taiwan (Hwang et al., 1984). Seedlots can be certified as pathogen free by indexing--growing out a random sampling and checking for diseased plants as clone to control lettuce mosaic virus (Grogan, 1980). The methodology used to produce pathogen-free planting material has virtually revolutionized the ornamental-plants industry (Baker and Linderman, 1979). This technology is now available to provide pathogen-free planting material for essentially all crops and to certify the planting materials as pathogen-free by highly sensitive serological methods. Such planting material sometimes costs more than the traditional material, but without good sanitation at this stage of growing a crop or starting an orchard, practices imposed at a later date may be of little value to the health and/or marketability of that crop.
Pest control by sanitation is accomplished mainly by physical and chemical methods, including sterilization of the surface of seeds or tools by heat or chemical-disinfectants, physical separation of disease-free tissue from infected plants or weed seeds from crop seeds, or physical removal of weeds or infested debris from an orchard or field. Biological control introduced on top of good sanitation can extend the period of pest and disease control started by sanitation but should not be used as a substitute for good sanitation practices (Cook and Baker, 1983).
Environmental and nutritional stresses on plants probably cannot be avoided completely during any given growing season, even with a good soil structure in the field and the use of a well-adapted cultivar. Examples of unavoidable environmental stress include chilling injury of a warm-temperature crop such as corn or soybeans, frost damage or winter injury of crops grown in the colder climates, high-temperature effects on cool-climate food crops grown in the tropics or subtropics, and water stress of crops grown in semi-arid regions. Nutritional stresses caused by clinical or subclinical deficiencies of macro- or micro-elements are also common and can vary with the growing season, stage of crop growth, and availability of nutrients released in the soil.
Some environmental stresses can kill plants outright, but more commonly they predispose plants to weak parasites and opportunistic pathogens. Deficiencies in almost any one of the macro- or micro-elements can then result in the plant becoming more susceptible to certain pests or diseases. Plants support large communities of microorganisms on or within their leaves and roots as epiphytes and endophytes. Nutritional relationships among these microorganisms range from strong and even obligate parasites (e.g. rust and powdery mildew fungi) to weak or facultative-type parasites (e.g. many Fusarium spp.), to strict saprophytes (e . g. most Cladosporium and Stemphylium spp.). With obligate parasites, the better the conditions for crop growth, the greater their activity and hence potential to cause damage (Baker and Cook, 1974). Biological control through use of plant genes for resistance to these parasites is the preferred method to limit their damage (see above). In contrast, the strict saprophytes associated with the living plant are essentially jockeying for position in preparation for the day when the plant is dead; they are then better positioned to take possession of the dead plant in advance of their would-be competitors. The weak parasites require only that the plant become stressed--almost dead-- in order to take more thorough possession of the tissues.
Weak parasites and opportunistic pathogens should not be interpreted as unimportant pathogens. Such plants without the weak parasites would probably recover once relieved of the stress but are not likely to recover once taken over by the pathogen. Moreover, pathogens that depend on an environmental or nutritional stress of their host to cause disease often have the ability to initiate or enhance the necessary stress. This can be illustrated by three root diseases of wheat favored, respectively, by wet soil, phosphorus deficiency, and water stress. Pythium root rot of wheat, like Pythium root rot of any other crop, is favored by wet soil. This pathogen begins its attack of wheat by infecting the embryos of germinating seeds during the first 1-2 days after planting (Hering et al., 1987). Some germinating seeds are killed by the infection but most emerge as stunted seedlings. Such seedlings use less water than healthy seedlings, hence the soil is almost always wetter where Pythium root rot occurs than where plants are healthy. Pythium root rot therefore can be the cause as well as the result of the wet-soil conditions. Take-all caused by Gaeumannomyces graminis var. tritici is favored by phosphorus deficiency of wheat (Reis et al., 1982). The parasitic attack of the roots by this pathogen limits the nutrient-absorbing ability of the wheat root system; the plant with missing roots is then even more likely to become phosphorus deficient and the disease develops all the more rapidly. Fusarium root rot caused by Fusarium culmorum is favored by plant waterstress (Papendick and Cook, 1974; see above), which this disease also causes by developing in the crowns and tiller bases, thereby increasing the resistance to water flow from roots to the tops of the plants.
Minimizing environmental or nutritional stresses can only be accomplished by careful crop management based on knowledge of climate and weather likely to occur in the area. Choice of planting date becomes especially critical for warm-temperature crops such as corn and soybeans, to avoid exposing these kinds of plants to chilling injury or other low-temperature stresses. Fusarium root rot or beans can be partially controlled by subsoiling, to loosen the soil and permit deeper penetration of the roots (Burke et al., 1972). The fertilization program for a crop should be developed and the precision improved through soil and/or tissue analysis for plant nutrients. It should also be pointed out, however, that since a significant number of the opportunistic pathogens favored by environment or nutritional stresses on the plant are also favored by the lack of rotation, the more frequently that crop rotation is used, the less critical the need to manage for environmental or nutritional stresses to achieve optimal plant health.
Beneficial organisms include mainly the naturel enemies of insect pests discussed in other papers in this series. Beneficial organisms also include the mycorrhizal fungi associated with plant roots and responsible for more efficient uptake of phosphorus and some protection of the host roots against certain soilborne pathogens (Marx, 1972; Schenck and Kellam, 1978), and the rhizobia responsible for symbiotic nitrogen fixation on roots of leguminous plants. Some cropping systems, and especially some forms of mixed cropping, can enrich populations of organisms useful in the protection or improvement of plant health and may therefore be potentially useful (Cook and Baker, 1983). Conversely, the use of fumigants applied to soils is likely to reduce populations of nontarget mycorrhizal fungi and rhizobia, and the use of fungicides or insecticides applied to the foliage can reduce populations of beneficial epiphytes or the naturel enemies of insect pests and should therefore be used only if necessary and then with caret
The large, diverse, and cosmopolitan communities of nonpathogenic microorganisms associated with plants, although probably among the first to thoroughly colonize the plants once dead, may in the meantime provide significant protection against plant disease (Blakeman and Fokkema, 1982) and insect attack (Siegel et al., 1987). The protection results from their ability to compete with pathogens on the plant surface or produce substances such as antibiotics and toxins inhibitory to pathogens or insect pests. The application of ice- strains or Pseudomonas syringae to protect plants against INA strains of this bacterium (Lindow, 1983), avirulent strain K84 of Agrobacterium radiobacter to protect roots against the crown-gall pathogen (Kerr, 1980), and antibiotic or siderophore (naturel iron-chelating compounds)-producing strains of Pseudomonas fluorescens to protect wheat roots against take-all (Weller and Cook, 1983) or Pythium root rot (Weller and Cook, 1987) all are examples of nonpathogenic, plant -associated microorganisms used to enhance a naturel biological control by increasing the population of these microorganisms on the plant.
This kind of protection is also afforded plants against herbivorous insects, as in the case of the fungal endophyte Sphacelia typhina associated with tall ryegrass. The presence of this endophyte was shown to cause fescue toxicosis (summer toxicity syndrome) in cattle allowed to graze on this plant, but while removal of the endophyte eliminated the risk to cattle, it also made the plants more susceptible to certain insect pests.
Many weed species and especially insect pests and diseases of perennial (e.g. Orchard) crops remain the most intractable to control by biological or physical methods and some can only be controlled economically by one or more synthetic pesticides. The system of holistic plant-health care proposed in this article does not preclude the use of synthetic chemicals. All too often, however, chemical controls are used instead of one or more of the first seven principles of plan health care outlined above, e.g. to permit the use of shorter or no crop rotation as a substitute for use of clean planting material, or to permit the use of a disease-susceptible cultivar when resistant cultivars are available. These kinds c choices are usually dictated by economics.
Pesticides should be considered but as the last and not the first step toward plant-health caret Some biological controls should also be considered as the last resort. The deliberate inoculation of plants with a mild strain of a plant virus to provide cross-protection against severe strains is an example and is use' today only where no alternative control exists (Cook and Baker, 1983).
Considerable progress has been made in recent years toward safer chemical pesticides and safer use of chemical pesticides. The selectivity of pesticides ha been improved markedly as a means to minimize nontarget effects. Some modern classes of herbicides are effective at greatly reduced rates--a few grams per hectare (fraction of an ounce per acre)compared with earlier classes of herbicides. The herbicide glyphosate, invaluable to conservation tillage systems, is quickly inactivated upon coming into contact with soil. Perhaps the greatest advance in safer use of pesticides is the introduction of monitoring, simulation models, artificial intelligence, and a systems approach as a means to improve the timing and limit the number of applications of pesticides. Each of these advances and approaches can be expected to improve in the future and therefore chemicals will continue to play an important role in plant-health care
Pest control is usually and correctly justified economically on the basis of the higher yield and presumably higher profit for the farmer. Healthy plants can also be justified sociologically on the basis of greater production of food. This was given emphasis in the theme of the 2nd International Congress of plant pathology held in Minneapolis, Minnesota in 1973--Healthy Plants Healthy People! Healthy plants can also help make our managed ecosystems more sustainable (Cook, 1986a & b). Plants with a large and healthy root system help anchor soil in place, which limits soil erosion. They also return more organic master to the soil and are more efficient in the uptake of mobile nutrients such as nitrates that otherwise might leach past the rooting zone and into the "round water. Healthy, vigorous plants are also more competitive with weeds, which can help reduce the need for herbicides. Healthy plants are also beneficial aesthetically.
1. Abel, P., R. S. Nelson, D. Barun, N. Hoffman, S. G. Rogers, R. T. Fraley, and R. N. Beachy. 1986. Delay of disease development in transgenic plants that express the tobacco mosaic virus coat protein gene. Science 232:738-743.
2. Allmaras, R. R., J. M. Kraft, and D. E. Miller. 1988. Soil compaction and crop residue effects on root health. Ann. Rev. Phytopathol. 26: In press.
3. Ashworth, L. J., Jr., D. P. Morgan, S. A. Gaona, and A. H. McCain. 1982. Polyethylene tarping controls Verticillium wilt in pistachios. Calif. Agric. 36(5-6):17-18.
4. Baker, K. F. 1962. Principles of heat treatment of soil and plant material. J. Aust. Inst. Agr. Sci. 28:118-126.
5. Baker, K. F. 1987. Evolving concepts of biological control of plant pathogens. Ann. Rev. Phytopathol. 25:67-85.
6. Baker, K. F., and R. J. Cook. 1974. Biological Control of Plant Pathogens, W. H. Freeman and Co, San Francisco, California. 433 pp. (Book, reprinted in 1982, Amer. Phytopathol. Soc., St. Paul, Minnesota).
7. Baker, K. F., and R. G. Linderman. 1979. Unique features of the pathology of ornamental plants. Ann. Rev. of Phytopathol. 17:253277.
8. Baker, R. 1985. Biological control of plant pathogens: Definitions. pp. 25-39. In M. A. Hoy and D. C. Herzog (eds.). Biological Control in Agricultural IPM Systems. Academic Press, New York, New York. 600 pp.
9. Beirner, B. P. 1967. Biological control and its potential. World Rev. Pest Control 6(1):7-20.
10. Biffen, R. H. 1906. Mendels law of inheritance and wheat breeding. J. Agric. Sci. 1:4-48.
II. Blakeman, J. P., and N. J. Fokkema. 1982. Potential for biological control of plant disease on the phylloplane. Ann. Rev. Phytopathol. 20:167-192.
12. Broadbent, P., and K. F. Baker. 1975. Soils suppressive to Phytophthora root rot in eastern Australia. pp. 152-157. In G. W. Bruehl (ed.). Biology and Control of Soil-borne Plant Pathogens. Amer. Phytopathol. Soc., St. Paul, Minnesota. 216 pp.
13. Burke, D. W., D. W. Miller, L. D. Holmes and A. W. Barker. 1972. Counteracting bean root rot by loosening the soil. Phytopathology 62:306-309.
14. Chet, I., and R. Baker. 1981. Isolation and biocontrol potential of Trichoderma hamatum from soil naturally suppressive to Rhizoctonia solani. Phytopathology 71 :286-290.
15. Cook, R. J. 1973. Influence of low plant and soil water potentials on diseases caused by soilborne fungi. Phytopathology 63:451-457.
16. Cook, R. J. 1980. Fusarium foot rot of wheat and its control in the Pacific Northwest. Plant Disease 64:1061-1066.
17. Cook, R. J. 1984. Root health: Importance and Relations to Farming Systems. In D. F. Bezdicek and J. F. Power (eds.). Organic Farming: Current Technology and Its Role in a Sustainable Agriculture. Amer. Soc. Agron. Special Publications: No. 46, Madison, Wisconsin, pp. 111-127.
18. Cook, R. J. 1985. Biological control of plant pathogens: theory to application. Phytopathology 75:25-29.
19. Cook, R. J. 1986a. Interrelationships of plant health and the sustainability of agriculture with special reference to plant diseases. Amer. J. Alter. Agri. 1:19-24.
20. Cook, R. J. 1986b. Plant health and the sustainability of agriculture, with special reference to disease control by beneficial microorganisms. Biol. Agric. and Horticulture 3:211-232.
21. Cook, R. J. 1986c. Wheat management systems in the Pacific Northwest. Plant Disease 70:894-898.
22. Cook, R. J., Chairman. 1987. Research Briefing Panel on Biological Control in Managed Ecosystems. Committee on Science, Engineering, and Public Policy, National Academy of Sciences, National Academy of Engineering, and Institute of Medicine. National Academy Press, Washington, DC. 12 pp.
23. Cook, R. J., and K. F. Baker. 1983. The Nature and Practice of Biological Control of Plant Pathogens. Amer. Phytopathol. Soc., St. Paul, Minnesota. 539 pp.
24. Cook, R. James, and A. D. Rovira. 1976. The role of bacteria in the biological control of Gaeumannomyces graminis by suppressive soils. Soil Biol. and Biochem. 8:269-273.
25. Cook, R. J., and D. M. Weller. 1987. Management of take-all in consecutive crops of wheat or barley. In I. Chet (ed.). Innovative Approaches to Plant Disease Control. John Wiley & Sons, Inc. pp. 41-76.
26. Cook, R. J., J. W. Sitton, and W. A. Haglund. 1987a. Increased growth and yield responses of wheat to reduction in the Pythium populations by soil treatments. Phytopathology 77:1192-1198.
27. Cook, R. J., D. M. Weller, and L. S. Thomashow. 1987b. Enhancement of root health and plant growth by rhizobacteria. In Molecular Strategies for Crop Protection. Alan R. Liss, Inc. pp. 125-134.
28. Corke, A. T. K. 1978. Interactions between microorganisms. Ann. Appl. Biol. 89:89-93.
29. Costa, A. S., and G. W. Muller. 1980. Tristeza control by cross protection: A U.S.-Brazil cooperative success. Plant Disease 64:538-541.
30. DeBach, P., ed. 1964. Biological Control of Insect Pests and Weeds. Reinhold, New York, New York. 844 pp.
31. Fulton, R. W. 1986. Practices and precautions in the use of cross protection for plant virus disease control. Ann. Rev. Phytopathol. 24:6781.
32. Gerlagh, M. 1968. Introduction of Ophiobolus graminis into new polders and its decline. Neth. Jour. Plant Pathol. 74:(Suppl. 2):1-97.
33. Grogan, R. G. 1980. Control of lettuce mosaic with virus-free seed. Plant Disease. 64:446449.
34. Hardison, J. R. 1976. Fire and flame for plant disease control. Ann. Rev. Phytopathol. 14:355-379.
35. Hering, T. F., R. J. Cook, and W.-h. Tang. 1987. Infection of wheat embryos by Pythium species during seed germination and the influence of seed age and soil matric potential. Phytopathology 77:1104-1108.
36. Hill, J. E. 1985. Methods for selecting beneficial Lactobacilli from swine. Advances in the Application of Microbiology to Agriculture. Pioneer Hi-Bred International, Inc., Microbial Genetics Division, Johnston, Iowa.
37. Holings, M. 1965. Disease control through virus-free stock. Ann. Rev. Phytopathol. 3:367-389.
38. Hwang, S. C., C. L. Chen, J. C. Lin. 1984. Cultivation of bananas using plantlets from meristem culture. HortScience 19:231-233.
39. Hyakumachi, M., and Tadao Ui. 1982. Decline of camping-off of sugar beet seedlings caused by Rhizoctonia solani AG-2-2. Ann. of the Phytopathol. Soc. of Japan. 48(5):600-606.
40. Jones, D., and M. E. Solomon, eds. 1974. Biology in Pest and Disease Control. Brit. Ecol. Soc. Symp. 13. 398 pp.
41. Katan, J. 1981. Solar heating (solarization of soil for control of soilborne pests). Ann. Rev. Phytopathol. 19:211-236.
42. Kerr, A. 1980. Biological control of crown gall through production of agrocin 84. Plant Disease 64:25-30.
43. Kuc, J. 1982. Induced immunity to plant disease. Bioscience 32:854-860.
44. Lindow, S. W. 1983. Methods of preventing frost injury caused by epiphytic ice nucleation active bacteria. Plant Disease 67:327-333.
45. Lundholm, B., and M. Stackerud, eds. 1980. Environmental Protection and Biological Forms of Control of Pest Organisms. Swedish Natural Science Research Council Ecological Bulletins 31:1 - 171.
46. Lupton, F. G. H. 1984. Biological control: The plant breeder's objective. Ann. Appl. Biol. 104:1-16.
47. Marx, D. H. 1972. Ectomycorrhizae as biological deterrents to pathogenic root infections. Ann. Rev. Phytopathol. 10:429-454.
48. McBeth, C. W., and A. L. Taylor. 1944. Immune and resistant cover crops valuable in root-knot-infested peach orchards. Proc. Amer. Soc. Hort. Sci. 45:158-166.
49. Miller, D. E., and D. W. Burke. 1975. Effect of soil aeration on Fusarium root rot of beans. Phytopathology 65:519-523.
50. Papavizas, G. C., and R. D. Lumsden. 1980. Biological control of soilborne fungal propagules. Ann. Rev. Phytopathol. 18:389-413.
51. Papendick, R. I., and R. J. Cook. 1974. Plant water stress and development of Fusarium foot rot in wheat subjected to different cultural practices. Phytopathology 64:358-363.
52. Payne, C. C. 1988. Pathogens for the control of insects--where next? In R. K. S. Wood and M. J. Way (eds.). Biological Control of Pests, Pathogens, and Weeds: Developments and Prospects. The Royal Soc. Lond. pp. 115-248.
53. Phillips, R. E., R. L. Bevins. G. W. Thomas, W. W. Freye, and S. H. Phillips. 1980. No-tillage agriculture. Science 208:1108-1113.
54. Reis, E. M., R. J. Cook, and B. L. McNeal. 1982. Effect of mineral nutrition on take-all of wheat. Phytopathology 72:224-229.
55. Rickman, R. W., B. Klepper, and R. K. Belford. 1985. Developmental relationships among roots, leaves, and tillers in winter wheat. In W. Day and R. K. Atkins (eds.). Wheat Growth Modelling. Plenum Publishing. pp. 83-98.
56. Riggs, R. D., D. A. Slack, M. L. Hamblen, and L. Rakes. 1980. Nematode control studies in soybeans. Ark. Agric. Exp. Sta. Report series 252. 32 pp.
57. Rishbeth, J. 1979. Modem aspects of biological control of Fomes and Armillaria. Eur. J. For. Pathol. 9:331-340.
58. Roelfs, A. P. 1988. Genetic control of phenotypes in wheat stem rust. Ann. Rev. Phytopathol. 26:351-367.
59. Rouse, D.1. 1988. Use of crop-growth models to predict the effects of disease. Ann. Rev. Phytopathol. 26:183-201.
60. Rovira, A. D., and A. Simon. 1982. Integrated control of Heterodera avenae. EPPO Bull. 12:517-523.
61. Russell, R. S. 1975. Plant root systems: their function and interaction with soil. McGraw Hill, London. 298 pp.
62. Schenck, N. C., and M. K. Kellam. 1978. The influence of vesicular arbuscular mycorrhizae on disease development. Florida Agric. Exp. Sta. Bull. 798 (Technical):1-16.
63. Schroth, M. N., and J. G. Hancock. 1982. Disease-suppressive soil and root-colonizing bacteria. Science 216:1376-1381.
64. Shipton, P. J. 1977. Monoculture and soilborne plant pathogens. Ann. Rev. Phytopathol. 15:387-407.
65. Siegel, M. R., G. C. M. Latch, and M. C. Johnson. 1987. Fungal endophytes of grasses. Ann. Rev. Phytopathol. 25:293-315.
66. Slack, S. A. 1980. Pathogen-free plants by meristem culture. Plant Disease 64:15-17.
67. Smucker, A. J. M., and A. E. Erickson. 1987. Anaerobic stimulation of root exudates and disease of peas. Plant Soil 99:423-433.
68. Stolzy, L. H., J. Letey, L. J. Klotz, and C. K. Labanauskas. 1965. Water and aeration as factors in root decay of Citrus sinensis. Phytopathology 55:270-275.
69. Templeton, G. D., R. J. Smith, and D. O. TeBeest. 1986. Progress and potential of weed control with mycoherbicides. Rev. Weed Sci. 2:1-14.
70. Vaeck, M., A. Reynaerts, H. Hufte, S. Jansens, M. De Bueckeleer, C. Dean, M. Zabeau, M. van Montagu, and J. Leemans. 1987. Transgenic plants protected from insect attack. Nature 328:33-39.
71. Waage, J., and D. J. Greathead. 1988. Biological control: challenges and opportunities. In R. K. S. Wood and M. J. Way (eds.). Biological Control of Pests, Pathogens, a Weeds: Developments and Prospects. The Royal Soc., London., pp 1-18.
72. Weinhold, A. R., J. W. Oswald, T. Bowman J. Bishop, and D. Wright. 1964. Influence of green manures and crop rotation on common scab of potato. Am. Potato Jour. 41:265-2'
73. Weller, D. M., and R. J. Cook. 1983. Suppression of take-all of wheat by seed treatments with fluorescent pseudomonads. Phytopathology 73:463-469.
74. Weller, D. M., and R. J. Cook.1987. Increase growth of wheat by seed treatments with fluorescent pseudomonads, and implications of Pythium control. Can. J. Plant Pathol. 8:328-334.
75. Wilson, C. L., and P. L. Pusey. 1985. Potential for the biocontrol of postharvest plant diseases. Plant Disease 69:375-378.
76. Zaag, D. E. van der. 1984. Reliability and significance of a simple method of estimating the potential yield of the potato crop. Potato Research 27:51-73.
Citation : Cook, James R., "Biological control and holistic plant-health care in agriculture", Vol. 3, No. 2 and 3, pp. 51-62.
Copyright © 1988
Reprinted with permission.
Info Request | Services | Become EAP Member | Site Map
Give us your comments about the EAP site
Ecological Agriculture Projects, McGill University (Macdonald
Campus)
Ste-Anne-de-Bellevue, QC, H9X 3V9 Canada
Telephone:
(514)-398-7771
Fax:
(514)-398-7621
Email: info@eap.mcgill.ca
To report problems or otherwise comment on the structure of this site, send mail to the Webmaster