JPR Index | Virtual Library | Magazine Rack | Search
By Caroline Cox
Biotechnology has the potential to quickly make profound changes in the use of pesticides by U.S. agriculture. While genetic engineering may seem like it belongs in futuristic novels, it is not just a fantasy of science fiction writers. Genetically engineered crop plants will be in farmers' hands in the next few years. We are therefore at a critical point where we need to evaluate this new technology and how it will impact our agricultural systems. What problems will the new technology bring? Will biotechnology be used to increase or decrease pesticide use? Will it move us towards or away from the goal of a sustainable agricultural system?
In theory, biotechnology could be used to prevent pest problems and thus reduce the need for pest management and pesticide use. Since the beginning of agriculture, plant breeders have developed crop varieties that were resistant to or tolerant of particular pests. For example, cotton varieties with long, twisted bracts (called frego bracts) around the bolls are resistant to boll weevil damage and solid stemmed wheat varieties are not damaged by the wheat stem sawfly. The tools of biotechnology could be used to make plant breeding easier and quicker.
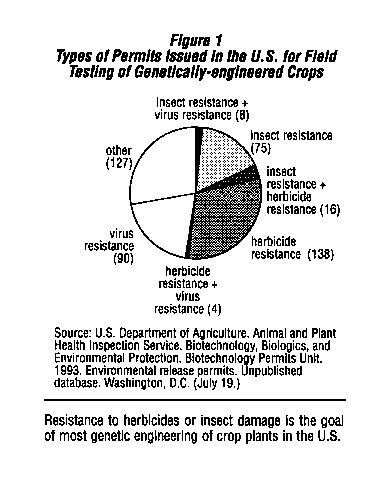
However, genetic engineering has c tended to move agriculture in the opposite direction, towards maintaining or increasing present pesticide use patterns. Almost 35 percent of the almost 500 permits for field tests of genetically engineered crop plants approved by the U . S. Department of Agriculture (USDA) since 1987 are for tests of plants that have been genetically engineered to be resistant to an herbicide.2 This means that the crop plant is~not damaged by the herbicide, so that it can be used to kill weeds growing with the crop without causing economic losses. The use of these genetically engineered crop plants will increase the use of the particular herbicide for which tolerance has been engineered.
Another 22 percent of the USDA permits are tests of crop plants that have been engineered to produce a microbial insect toxin ,2 and thus increase the use of these insecticidal compounds. (See Figure 1.)

This article is an introduction to the issues important when considering the interaction between pesticide use and biotechnology. In particular, emphasis will be on the questions that need to be asked about the development and use of crop plants with genetically-engineered herbicide resistance and microbial insect toxins.
Genetic engineering of herbicide resistance in crop plants is now big business. Of the 158 permits issued by USDA for field testing of herbicide resistant crops, 74 have been issued to large pesticide manufacturers and 35 have been issued- to large seed companies.2 3 Almost 80 percent of the field tests involve three major crops (cotton, corn, and soybeans) and tests have been conducted in 31 states. (See Figure 2.)
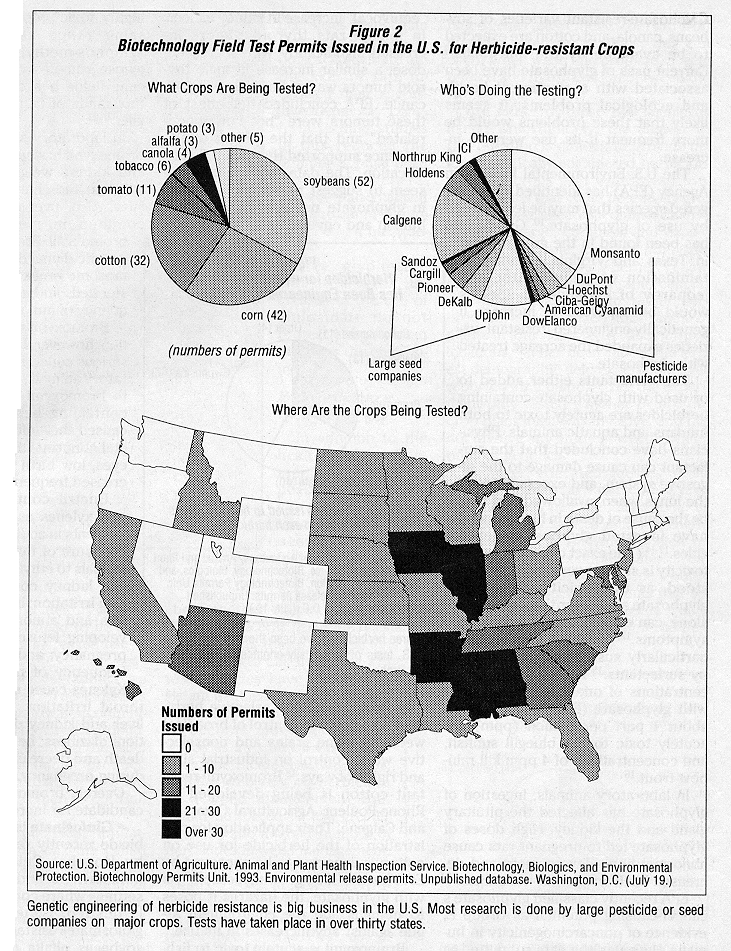
Cotton is grown on almost 10 million acres in the U.S., soybeans on nearly 60 million acres, and com on almost 70 million acres. Together, these three crops occupy about 30 percent of the arable land in the U.S. 4 5 Any changes made on herbicide use on these crops will have large-scale impacts on the agricultural economy, environmental quality, and human health.
Clearly, corporations involved in producing or selling seeds and pesticides see the potential for large profits. Industry consultants have estimated sales of genetically-engineered seed in the U.S. will reach almost 7 billion dollars by the end of this decade ,6 with the accompanying sales of herbicides adding to the size of the potential market. When farmers use a crop variety engineered to be resistant to a particular herbicide, they are almost certain to purchase and use the herbicide to which the crop is tolerant and thus the manufacturer of the herbicide is guaranteed to increase its market share.
In addition to taking advantage of these potential profits, the biotechnology industry argues that it will help reduce agriculture's environmental impacts. Since most of the acreage of major U.S. crops is already treated with herbicides, genetic engineering of herbicide resistance will cause farmers to switch from one herbicide to another rather than actually increasing the number of acres treated. Herbicides are already used on 96 percent of U.S. com acreage, for example, 88 percent of U.S. cotton acreage, and 97 percent of U.S. soybean acreage.7 This switch, argue biotechnologists, is from older herbicides that have damaged human health or the environment to newer herbicides with "desirable environmental characteristics."8
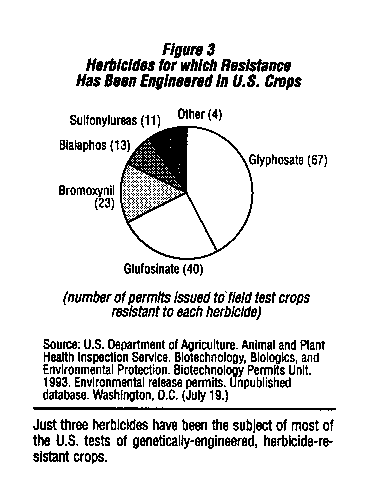
These arguments must be considered with caution. The first step in a careful analysis of the impacts of agricultural biotechnology is to look at the herbicides whose use will increase as the first generation of genetically engineering crops becomes commercially available. Based on~the number of permits for field tests issued by USDA, these herbicides will be glyphosate, glufosinate, and bromoxynil which together account for over 80 percent of the field tests conducted so far. Rather than having "desirable environmental characteristics,"8 all three have significant problems associated with their use. It quickly becomes clear that all three were selected because genes were available to engineer resistance and because of their potential for increased sales, rather than the lack of health and environmental concerns.
Glyphosate is currently used as a broad-spectrum herbicide in agriculture, forestry, and urban settings. Between 15 and 20 million pounds are now used annually in the U.S.
Glyphosate-resistant varieties of soybeans, canola, and cotton are expected to be available by the mid-1990s.9 Current uses of glyphosate have been associated with a number of health and ecological problems; it seems likely that these problems would be more frequent if its use were to increase.
The U.S. Environmental Protection Agency (EPA) has identified 76 endangered species that may be jeopardized by use of glyphosate. Glyphosate has been found in the groundwater in Texas and Virginia.Both contamination of groundwater and jeopardy of endangered species would be expected to increase if genetically-engineered resistant varieties expanded the acreage treated with glyphosate.
The surfactants either added to or used with glyphosate-containing herbicides are acutely toxic to both humans and aquatic animals. Physicians have concluded that the surfactant can cause damage to the digestive system, and excess fluids in the lungs when swallowed and may be the cause of death in humans who have ingested glyphosate herbicides. (The exact cause of human toxicity is still not completely understood, as the toxicity of neither glyphosate alone, nor the surfactant alone, can explain human poisoning symptoms. Gilled animals are particularly susceptible to damage by surfactants. For example, concentrations of one surfactant used with glyphosate (Entry II) as low as about 1 part per million (ppm) are acutely toxic to the bluegill sunfish! and concentrations of 4 ppm kill rainbow trout.
In laboratory animals, ingestion of glyphosate has affected the pituitary gland and the kidney. High doses of glyphosate fed to pregnant rats cause abnormal bone development and decreased birth weights of the babies.
EPA recently classified glyphosate cancer-causing potential as Group E. evidence of noncarcinogenicity in humans. However, the data submitted to EPA by Monsanto, the manufacturer of glyphosate, in support of this classification show that in male rats glyphosate caused a significant increase in pancreatic tumors at two doses, a significant increase in river tumors with increasing dose, and an equivocal" increase in kidney cancer. In female rats thyroid tumors increased significantly with increasing dose; a similar increase in male thyroid tumors was of borderline significance. EPA concluded that most of these tumors were not compound-related" and that the weight of the evidence supported the Group E classification. The data, however, hardly seem to suggest that large increases in glyphosate use are protective of human and environmental health.
Bromoxynil is a selective herbicide now used for control of broadleaf weeds in some grains and non-selective weed control on industrial sites and rights-of-ways. Bromoxynil-resistant cotton is being developed by Rhone-Poulenc Agricultural Company and Calgene. Their application for registration of the herbicide for use on cotton is expected to be approved by EPA for the 1994 growing season. As with glyphosate, the hazards it poses to humans and the environment argue against any increases in its use.
Bromoxynil is acutely toxic to fish. Concentrations of 23 parts per billion (ppb) are toxic to catfish and 50 ppb to rainbow trout. Other aquatic organisms are also killed by bromoxynil; water fleas are killed by concentrations of just over 100 ppb. This acute toxicity to fish is classified as very highly toxic by EPA and is in the same range as the toxicity of , azinphos-methyl, the insecticide whose rue-off from Louisiana sugar cane fields has caused hundreds of thousands of fish to die in repeated kills
In laboratory animals, ingestion of bromoxynil has caused increased river and kidney weights, reduced body Weights, and changes in blood chemistry. Also, river cancers were more common in male rats ingesting bromoxynil. Bromoxynil also causes genetic damage; it has caused chromosome breakage in Chinese hamster cells and an increase in the frequency of mutations in mouse cells.
Bromoxynil effects on reproduction, however, have caused the most serious concerns. In studies of laboratory animals, exposure of mothers to bromoxynil or the bromoxynil-containing herbicide Buctril has caused their offspring to have skeletal abnormalities, missing or small eyes, low birth weights, and an increased frequency of fetal death.22
Buctril contains ethylbenzene and xylenes as so called inert" ingredients in addition to bromoxynil. Exposure of humans or laboratory animals to ethylbenzene causes lung and kidney congestion; dizziness; skin irritation; incoordination; extra ribs and abnormal kidneys in developing fetuses; fetal loss during pregnancy; and an increase in the frequency of malignant tumors.25 Xylenes cause skin, eye, nose, and throat irritation; impaired memory; river and kidney damage; incoordination; dizziness; hearing loss; and fetal death and decreased fetal weight gain during pregnancy.26
Overall, bromoxynil is not a good candidate for increased use.
Glufosinate is a nonselective herbicide recently registered in the U.S. by Hoechst Celanese for use by homeowners and in light industrial settings where complete weed control is desired. Genetically engineered glufosinate-resistant varieties of com, soybeans, alfalfa, canola, rice, and cucumber have been field tested in the U.S. by a number of different companies. Because it is a new herbicide (registered in the U.S. in July, 1993), little information about its health or environmental effects is available, however the tests submitted in support of its registration identify a number of problems that need to be considered in evaluating increased use of this herbicide.
Glufosinate and its degradates are "mobile and persistent." This indicates the potential for groundwater contamination, and EPA has required a groundwater advisory statement on the label of the glufosinate-containing product, Ignite. Ignite is also toxic to fish and concentrations of less than 30 ppm kill rainbow trout.27
Glufosinate kills plants by inhibiting the activity of an enzyme called glutamine synthetase which is involved in both the detoxification of ammonia aria the metabolism of amino acide. Glufosinate inhibits the same enzyme in mammals and reduces glutamine levers in the river, braie, and kidneys.
In laboratory animals, glufosinatecontaining herbicide products are irritating to both eyes and skin. Feeding glufosinate increases kidney weights in rats; increases river weights and blood potassium levers in mice; and decreases weight gain and thyroid weights in dogs. Glufosinate on the skin of rats increased their aggressive behavior. Long-term feeding studies in rats reduced the number of pups born during two generations and also caused an increase in the incidence of a birth defect.27
Like glyphosate and bromoxynil, increases in the use of glufosinate do not protect either human health or the environment.
In addition to the health and environmental problems posed by the herbicides to which resistance is being engineered into crop plants, engineering of resistance genes has heightened concerns about another difficult problem. This problem concerns herbicide resistance in the weeds that the herbicides are being used to kill. Currently 273 weed species have become resistant to at least one herbicide.23 If herbicide resistance genes were to become more common in weeds as a result of widespread use of herbicide-resistant crops, farmers who rely on herbicides as a weed management tool would be forced to use greater amounts and a larger number of herbicides in weed management.
Herbicide resistance genes in weeds could increase in frequency because of the use of herbicide-tolerant crops by two different mechanisms. First, use of a particular herbicide on a crop creates strong selective pressure for any resistance genes in the weed population. If any resistant individuals are present, they will survive the herbicide treatment and their populations will increase rapidly.8 Second, resistance genes might move from the crop plant to weed populations; this can occur when weed and crop are closely related and hybridization between the two occurs.29
Are either of these two scenarios likely? Unfortunately, the answer to this question is difficult to provide before genetically-engineered herbicide-resistant crops gain widespread use. Experiments that could potentially give us an answer ahead of commercial use would be large, complex, and expensive. For example, a recent study d~ signed to determine whether genetically-engineered oilseed rape (canola) was more "invasive" than traditionally bred varieties utilized thousands of plants in four habitats in each of three locations.30 It has been called "the largest demographic field experiment ever reported for a plant. Yet, the experiment only tested whether or not the crop plant itself was invasive, and did not involve any study of weed populations or genetics.
However, educated guesses about these scenarios are not comforting. Biotechnology has been used so far to transfer traits with a simple genetic mechanism. The enzyme targeted by glyphosate, for example, differs from a resistant form of the enzyme by only a single amino acid.8 It seems that selection for resistance in weeds would therefore not be surprising if the herbicide is widely used.
Crop/weed hybridization has already occurred between conventionally bred crops and their associated weeds. Geneticists and weed scientists have studied a number of examples: hybridization between cultivated corn and wild relatives, 32 and between cultivated squash and its wild relatives.33 Hybridization between cultivated sorghum and the closely related weed species johnson grass, "one of the world's worst weeds,"29 has been implicated in the evolution of particularly aggressive varieties of the weed.29 Wild oats often grow in cultivated oat fields.34
Clearly, this kind of hybridization will cause the most potential problems where a genetically engineered crop is grown in regions where it has close relatives. For many crops! these regions are in tropical countries. However, this kind of situation `also exists in North America. For example, eight of California's ten major vegetable crops have wild relatives that are either the same species or a closely-related species.29
As the National Research Council wrote in its discussion of pesticide resistance, "Resistance to pesticides is a global phenomenon. It is growing in frequency and stands as a reminder of the resiliency of nature. The potential interaction between biotechnology and the development of herbicide resistance in weed species is a subject that cannot be ignored.
Concerns about the development of resistance have also been at the center of discussions about attempts to genetically engineer crop plants to produce chemicals that are toxic to insects.
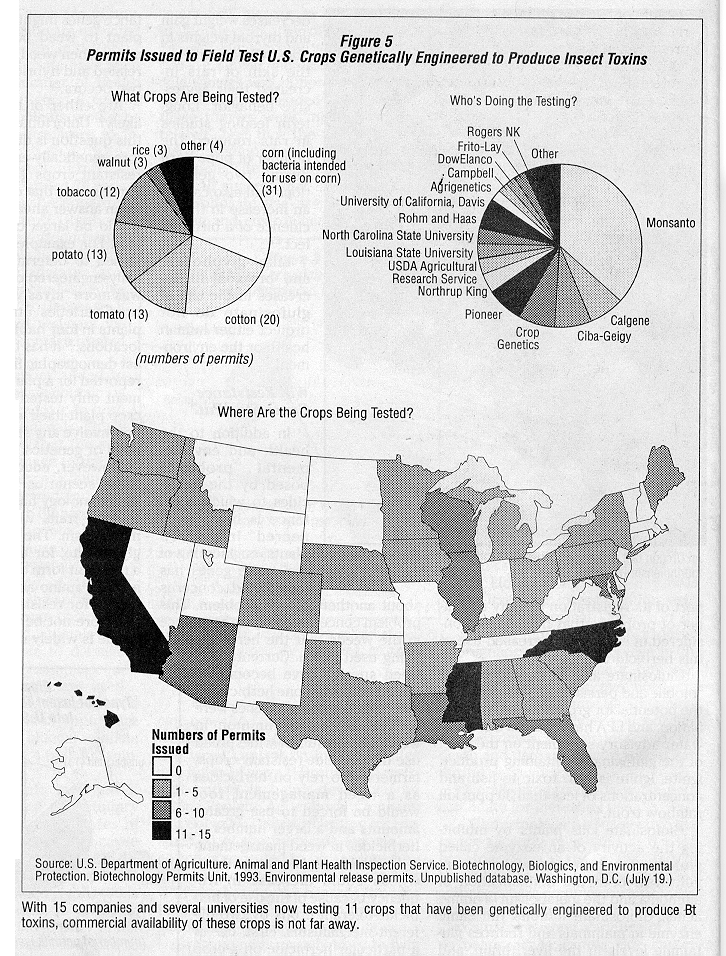
Most of the field test permits issued by USDA for crops that have been genetically engineered to be insect resistant contain a gene from the bacteria Bacillus thuringiensis (Bt) which produces an insect toxin.2 (See Figure 4.) The toxins are not all the same; biotechnologists have described 29 different toxins from different strains of the bacteria.36

Because it has some desirable ecological characteristics and has fewer impacts on human health than chemical insecticides, Bt is widely used by organic growers and in integrated pest management programs. In some situations, either applied as an insecticide or produced through genetic engineering, it can dramatically decrease the amount of conventional insecticides used by farmers. For example, researchers working in Oregon with potato plants genetically engineered to produce a Bt toxin that kills the Colorado potato beetle found that insecticides were not needed to control a second potato pest, the green peach aphid. The aphid populations were kept below economically damaging levels by predatory insects and spiders. The predators, killed by conventional insecticides, were not affected by the Bt toxin.37
If the toxin were to be produced by genetically engineered crops on a large scale, however, it seems likely that insect pest species could rapidly develop resistance to the toxin.38 This would end its usefulness in alternative and conventional agriculture and potentially increase the numbers and amounts of insecticides used by farmers. (See "Bacillus Thuringiensis: Industry Frenzy and a Host of Issues," JPR 9(1):18~21.) With 15 companies and several universities now field testing 11 different crop species that have been genetically engineered to produce Bt toxins in 30 states, 2 (see Figure 5) commercial availability of crops that produce Bt toxins is not far away.
Concerns about insects developing resistance to insecticides are not new, nor are they restricted to genetically-engineered insecticides. There are now over 600 species of insects known to be resistant to at least one insecticide.39 In addition, insects as a group seem remarkably capable of developing resistance to toxic chemicals and populations of these insects spread quickly. For example, Richard French-Constant, an entomologist: at the University of Wisconsin, Madison, has identified the mutation that makes fruit flies resistance to cyclodiene insecticides. It is caused by "a single change in the chain of nucleotide bases that make up the flies DNA. 39 The mutation appears to have arisen in one location and spread rapidly around the world.39
Crop plants that have been genetically engineered to produce the Bt toxin, however, potentially set up a situation that is optimal for allowing resistance in the pest insects to develop. This is true for two principal reasons:
Simple Genetic Mechanisms. When plants are resistant to insect attack, and that resistance is controlled by a simple genetic mechanism, the evolution of resistance in the insects is common. In the language of plant breeders this is called vertical resistance and is "generally believed to be short-lived because with a single gene conditioning resistance, only a single gene mutation in the insect is required to overcome it. It requires the addition of only a single gene to a crop plant to produce the Bt toxin, thus resistance in the insect pests can also be easily accomplished. Evidence for this comes from both field and laboratory; field resistance to Bt toxins has been reported in Hawaii 40 and laboratory studies indicate that resistance to the toxin produced by one strain of Bt can~ give insects cross-resistance to other Bt strains
· Persistent Exposure to High Toxin Levels. Integrated pest management (IPM) techniques use intensive pest suppression only when pests are abundant enough to cause economic damage. This not only saves farmers money and prevents unnecessary pest control measures, but also helps to prevent the development of insect resistance. The use of genetically-engineered crops producing Bt toxins, however, does not allow for this approach. Farmers plant resistant seed before they know whether or not they have a pest problem. The plants then produce toxin whether or not the toxin is needed for pest control and do so for the entire length of the growing season and throughout all plant tissues. This persistent and uniform exposure of the pest insect to the toxin creates a strong selective pressure for the pest to evolve resistance to the Bt toxin.38
Biotechnologists are experimenting with various methods of reducing this persistent and uniform exposure. For example, scientists at Ciba-Geigy Corporation have tested a proprietary chemical that can trigger production of the Bt toxin by special genetically-engineered tobacco plants.42 If made available on a commercial scale, farmers could use such a product to trigger Bt production when needed for pest management. The potential impacts of t he proprietary chemical on human and environmental health are unknown.) Other Ciba-Geigy scientists have used a genetic promoter in corn to produce high levels of the Bt toxin in leaves and pollen (consumed by the pest species, the European com borer) and low levels in other parts of the plant (consumed by humans and livestock).43 These techniques may delay the development of resistance in insect pests, but cannot eliminate it.
The use of crops genetically engineered to produce Bt toxins has the potential to expand rapidly. Field tests of these crops have been conducted on two major crops, com and cotton, as well as a variety of minor crops.2 (See Figure 5.) ln addition, Bt toxin genes have beep engineered into poplar and spruce; thus, forestry uses of trees with genetically-engineered Bt toxins are on the horizon.244 Strains of Bt with new toxins continue to be discovered, and some researchers fee! that "it may be possible to find Bt strains specific for virtually any pest target,"36 from single-celled microbes to flatworms, nematodes and arthropods. Each new use makes the concerns about resistance more compelling.
U.S. agriculture has become increasing dependent on inputs of synthetic chemicals during the last fifty years. The results of this dependency are now becoming clear: agricultural pesticides are in groundwater, rivers, and air; farmers and farm-workers suffer both acute and chronic health problems due to their exposure; and consumers become increasingly concerned about the pesticide residues on their food.45 What we need as we enter the twenty-first century is a new vision of how agriculture can work in this country, one that will produce food with truly low-input and sustainable techniques. This will come to pass only if we invest now in the research that is needed to develop appropriate management techniques. Biotechnology, unfortunately, is not now oriented in this direction.
Agricultural research priorities in the U. S. are set mostly by corporations that provide farmers with the inputs (seeds, fertilizers, and pest management products) farmers need to produce their crops. When there is a conflict between short-term profits and long-term sustainability it is not easy for a corporation to neglect its own economic stability in favor of society's future benefits. The results are, as Hope Shand director of the Rural Advancement Fund International wrote, "deeply disappointing," "Instead of durable pest resistance, the industry focuses on short-term pesticide tolerance. Rather than concentrating on sustainable agriculture, companies engineer proprietary vegetables with extended shelf life."46 Only a fundamental change in research priorities will this disappointment change to hope.
References
1. Callun, R.L. and C.S. Khush. 1980. Genetic factors affecting expression and stability of resistance. In Maxwell, F.C. and P.R. Jennings. (eds.) Breeding plants resistant to insects. New York, NY: John Wiley and Sons.
2. U.S. Department of Agriculture. Animal and Plant Health Inspection Service. Biotechnology, Biologics, and Environmental Protection. Biotechnology Permits Unit. 1993. Environmental release permits. Unpublished database. Washington, D.C. (Jury 19)
3. Coldburg, R. et al. 1990. Biotechnology's bitter harvest: Herbicide-tolerant crops and the threat to sustainable agriculture. The Biotechnology Working Croup. (September.)
4. Food and Agriculture Organization of the United Nations. 1992. Production yearbook. FAO Statistics Series No. 104. Rome, Italy.
5. Tarrant, J. (ed.) 1991. Farming and food. New York, NY: Oxford University Press.
6. Kloppenburg, J.R 1988. First the seed: The political economy of plant biotechnology, 1492-2000 Cambridge, U.K.: Cambridge University Press.
7. U.S. Department of Agriculture. National Agricultural Statistics Service. Agricultural Statistics Board. 1993. Agricultural chemical usage: 1992 field crops summary. Washington, D.~. (March.)
8. Krlmsky, S. and Wrubel, R. 1993. Agricultural biotechnology: An environmental outlook. Medford, MA: Tufts University Department of Urban and Environmental Policy.
9. CRC Press, Inc. 1993. Herbicide-tolerant crops controversy aired at AAAS meeting. Pesticide and Toxic Chemical News (February 17): 20-21.
10. U.S. EPA. Office of Pesticide Programs. 1986. Guidance for the reregistration of pesticide products containing glyphosate as the active ingredient. Washington D,C. (dune) ,
11. U.S. EPA. Office of Pesticide Programs. Environmental Fate and Effects Division. Environmental Fate and Groundwater Branch. 1992. Pesticides in "round water database. A compilation of monitoring studies: 19711991. National summary. Washington, D.C. (September)
12. Sawada, Y.Y. et al. 1988. Probably toxicity of surface-active agent in commercial herbicide containing glyphosate. Lancet 1(8580):299.
13. Tominack, R.L. 1991. Taiwan national poison center survey of glyphosate-surfactant herbicide Ingestions. Clin. Toxicol 29(1):91109.
14. Talbot, A.R. 1991. Acute poisoning with a glyphosate-surfactant herbicide ('Roundup'): A review of 93 cases. Human Exp. Toxicol 10:1-8.
15. Rankin, J.C., R.M. Stagg, and L. Bollis. 1982. Effects of pollutants on gills. In Houlihan D..F., J.C. Rankin, and T.J. Shuttleworth (eds.). Cills. Cambridge, U.K.: Cambridge University Press.
16. Monsanto Company. 1989. Entry 11 Surfactant material safety data sheet. St. Louis, MO. (June.)
17. Dykstra, W. and Chali, G.Z. 1991. Second peer review of glyphosate. Memo to R. Taylor and L. Rossi. Washington, D.C. U.S. EPA. Office of Pesticides and Toxic Substances. Health Effects Division. (October 30.)
18. Weed Science Society of America. 1983. Herbicide handbook. Fifth Edition. Champaign, IL.
19. U.S. EPA. Office of Pesticide Programs. 1980. Guidance for the reregistration of pesticide products containing azinphos-methyl as the active ingredient. Washington, D.C. (September 11.)
20. Marcus,F.F. I991.Louisiana baffled by vast fish kills. The New York Times (August 21):A13.
21. U.S. EPA. Communications, Education, and Public Affairs. 1993. EPA reaches agreement with registrants on risk-reduction measures for use of pesticide azinphos-methyl on sugarcane in Louisiana. EPA Press Advisory. (April 23.)
22. California Department of Food and Agriculture. Medical Toxicology Branch. 1990. Summary of toxicology data: Bromoxynil octanoate. Sacramento, CA. (dune 6.)
23. Rogers, J.M. et al. 1991. Developmental toxicity of bromoxynil in mice and rats. Fund Appl Toxicol 17: 442447.
24. MSDS reference for crop protection chemicals. Fourth edition. 1992. New York: Chemical and Pharmaceutical Press.
25. U.S. Department of Health and Human Services. Public Health Service. Agency for Toxic Substances and Disease Registry. 1990. Toxicological profile for ethylbenzene. Washington, D.C.
26. U.S. Department of Health and Human Services. Public Health Service. Agency for Toxic Substances and Disease Registry. 1990. Toxicological profile for total xylenes. Washington, D.C.
27. U.S. EPA. Office of Pesticides' and Toxic Substances. 1993. Pesticide fact sheet: Glufosinate ammonium. Washington D.C. (dune 29.) .
28. LeBaron, H.M. and J. McFarland. 1990. Herbicide resistance in weeds and crops. In M.B. Green, H.M. LeBaron, and W.K. Moberg (eds.). Managing resistance from fundamental research to practical strategies. Washington, D.C. American Chemical Society. Pp.336-352.
29. E}lstrand, N.C. and C.A. Hoffman. 1990. Hybridization as an~avenue of escape for engineered genes: Strategies for risk reduction. Bioscience 40(6):438442.
30. Crawley, M.J. et al. 1993. Ecology of transgenic oilseed rape in naturel habitats. Nature 363(6430): 620-623.
31. Kareiva, P. 1993. Transgenic plants on trial. Nature 363(6430):580-581.
32. Doebley, J. 1990. Molecular evidence for gene flow among Zea species. Bioscience 40(6):443448.
33. Watson, H.D. 1990. Gene flow in squash species. Bioscience 40(6):449455.
34. Cressel, J. 1992. Indiscriminate use of selectable markers - sowing wild oats? Trends in Biotechnology 10:382.
35. National Research Council. Board on Agriculture. Committee on Strategies for the Management of Pesticide Resistant Pest Populations. 1986. Pesticide resistance: Strategies and tactics for management. Washington, D.C.: National Academy Press.
36. Feitelson, J.S., J. Payne, and L. Kim. 1992. Bacillus thuringiensis: Insects and beyond. Bio/Technology 10:271-275.
37. Reed, C.L. 1993. The effect of Colorado potato beetle control measures on non-target arthropode. Oregon Potato Commission Research Progress Reports. Pp. 4-17.
38. Could, F. 1988. Evolutionary biology and genetically engineered crops. BioScience 38(10:26-33.
39. Wuethrich, B. 1993. Globe-trotting insects spread resistance. Science News (dune 12):373.
Tabashnik, B.E. et al. 1990. Field development of resistance to Bacillus thuringiensis in diamondback moth (Lepidoptera: Plutellidae). J. Econ. Ent. 83(5): 16711676.
41. Could, F. 1992. Broad-spectrum resistance to Bacillus thuringiensis toxins in Heliothis virescens. Proc. Natl Acad Sci. 89:7986-7990.
42. Williams, S. et al. 1992. Chemical regulation of Bacillus thuringiensis ~endotoxin expression in transgenic plants. Bio-Technology 10:540-543. (May.)
43. Koziel, M.C. et al. 1993. Field performance of elite transgenic maize plants expressing an insecticidal protein derived from Bacillus thuringiensis. Bio/Technology 11:194200. (February 11.)
44. Cupta, P.K. et al. 1993. Forestry in the 21st century. Bio/Technology 11:454459. (April.)
45. Henao, S. et al. 1993. Pesticides and health in the Americas. Washington, D.C.: Pan American Health Organization. World Health Organization. Division of Health and Environment.
46. Shand, Hope. 1993. Editorial 2003: Agbio and Third World Development. Bio/Technology 11:S13. (March.)
Citation for this article: Cox, Caroline. 1993, "Biotechnology and agricultural pesticide use : an interaction between genes and poisons", Vol. 13, No. 3, Fall 1993, pp. 4-11
Copyright © 1993 Northwest Coalition for Alternatives to Pesticides.
Reprinted with permission.
Info Request | Services | Become EAP Member | Site Map
Give us your comments about the EAP site
Ecological Agriculture Projects, McGill University (Macdonald
Campus)
Ste-Anne-de-Bellevue, QC, H9X 3V9 Canada
Telephone:
(514)-398-7771
Fax:
(514)-398-7621
Email: info@eap.mcgill.ca
To report problems or otherwise comment on the structure of this site, send mail to the Webmaster