JPR Index | Virtual Library | Magazine Rack | Search
By Mary O'Brien
"In general, [human health] research demonstrates that pesticide poisoning can lead to poor performance on tests including intellectual functioning, academic skills, abstraction, flexibility of thought, and motor skills; memory disturbances and inability to focus attention; deficits in intelligence, reaction time, and manual dexterity; and reduced perceptual speed Increased anxiety and emotional problems have also been reported "
United States Congress Office of Technology Assessment, Neurotoxicity: Identifying' and Controlling Poisons of the Nervous Systems
Students learn by using their central nervous system, assisted by a healthy body, adequate nutrition, positive sense of well-being, fine teachers (both inside and outside of school), and a clean environment. Pesticide exposure, however, robs a student of a clean environment, can undermine or destroy the student's health, and may directly affect the student's central nervous system. Learning then becomes another casualty of pesticides.
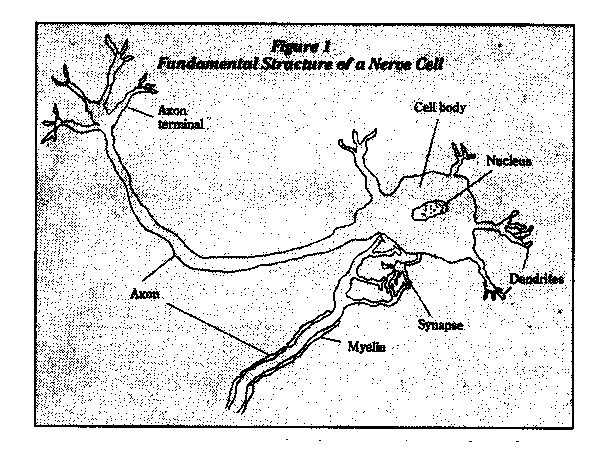
A human's brain and spinal cord (central nervous system) control vision, hearing, speech, learning, memory, and muscular movements. All of these functions are based on the fundamental unit of the nervous system, the nerve cell, or neuron (Figure 1). Electrical nerve impulses travel along the axons and dendrites of the nerve cell and the cell synthesizes and secretes neurotransmitters, specialized chemical messengers that interact with receptors of other neurons to provide communication.
Glial cells appear to support neurons, with certain of them producing myelin, a fatty substance that covers the axons of many neurons and allows the electrical nerve impulses to travel farther and faster than they otherwise could.
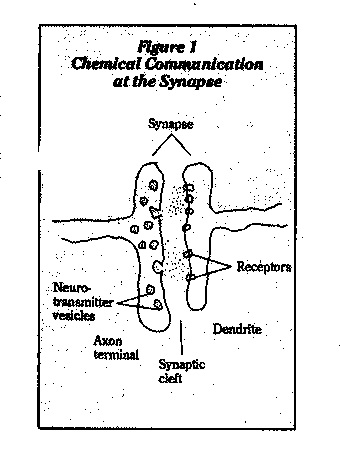
The point of interaction between neurons is the synapse (Figure 2). Neurotransmitters stored at the tips of the axon are released by electrical impulses, travel across the synaptic space to the next axon, where they bind to receptors and trigger biochemical events that lead to electrical excitation or inhibition. A nerve impulse is thereby passed on or halted.
The peripheral nervous system (nerves that travel to and from the spinal cord, sense organs, glands, blood vessels, and muscles) is more vulnerable than the central nervous system to neurotoxins... "
Different neurotoxic chemicals affect different sites: neurons, glial cells and myelin, the neurotransmitter system, and blood vessels supplying the nervous system.
Most of the central nervous system is partially protected from toxins by the blood-brain barrier, a layer of cells in blood vessel walls that allows some substances to pass into the nerve tissue and prevents others from doing so. Small compounds and compounds that are soluble in lipids (e.g., fat), tend to cross this barrier more easily than larger or water soluble compounds. The brain is particularly vulnerable to lipophilic toxins (those attracted to components of cells that are not soluble in water) since 50 percent of the dry weight of the brain is lipid; other organs of the body are 6 to 20 percent lipid.
The peripheral nervous system (nerves that travel-to and from the spinal cord, sense organs, glands, blood vessels, and muscles) is more vulnerable than the central nervous system to neurotoxins because it lies outside the central nervous system.
The developing nervous system of a fetus or infant, however, is especially vulnerable to certain toxins. Its cells are growing, dividing, moving around, and making connections, and the blood-brain barrier is incomplete.~' While exposure to neurotoxins during the early part of fetal development may result in spina bifida (exposed vertebral column) and anencephaly (absence of part or all of the brain), later development leaves the cerebrum and cerebellum (portions of the brain responsible for sight and movement) particularly vulnerable.
Neurotoxic substances may also affect cells of the immune system, which can in turn influence nervous system functioning. Recent research in this area has led to a new field of research known as neuroimmunology.
The liver is the body's principal organ of detoxification' with the kidney, intestine and lung also playing major roles. Once in the human body, toxic substances often undergo biotransformation in the liver, with the intestine, kidney, and lungs also playing major roles. Biotransformation usually changes lipophilic compounds to water soluble compounds so that they are more easily excreted. In the process, it may yield compounds that are more toxic. As a result, the compound originally entering an organism may not be the toxin that eventually acts on the nervous system.
Likewise, chemical interactions may occur among multiple toxic substances, causing additive effects (i.e., the combined effects are equal to the sum of the effects of each of the substances individually) or synergistic effects (i.e., the combined adverse effects exceed the sum of the individual effects). Potentiation occurs when a substance that is not toxic increases the toxicity of another substance.
Very few suspected neurotoxic chemicals have been evaluated in the laboratory, and even fewer have been thoroughly tested. Under the nation's pesticide law, the Federal Insecticide, Fungicide and Rodenticide Act (FIFRA), neither pesticide formulations nor individual pesticide ingredients need be tested for neurotoxicity (with the exception of a single delayed peripheral neuropathy test required for organophosphate non-secret (active) ingredients.3
Research provides evidence that certain pesticide ingredients can and do attack learning by à variety of mechanisms. A brief look at some of this research on organophosphate and carbamate pesticides, secret solvent ingredients, and dioxin contaminants of pesticides follows.
Organophosphate and carbamate pesticides and nerve gases act as neurotoxins by inhibiting acetylcholinesterase, the enzyme that inactivates the neurotransmitter acetylcholine (Figure 2). This creates a buildup of acetylcholine, which causes nervous system dysfunction.
Acute exposures to organophosphate pesticides have been shown in some cases to cause apparently permanent intellectual damage, and low level, chronic exposures to organophosphate and carbamate pesticides can result in accumulated inhibition of acetylcholinesterase to the point of acute effects. Moreover, low-level, nonchronic exposure has been shown in some cases to lead to behavioral effects before inhibition of acetylcholinesterase is measurable.
Vision and organophosphates Japan, a heavy user of organophosphate pesticides, experienced a tremendous increase of cases of myopia (nearsightedness) beginning several years after World War 11.4 Three distinct peaks in incidence were observed in 1962-1965, 1969, and 1973. The amount of organophosphates used increased during the same period in parallel fashion, with a one-year time lag in myopia incidence in 1969 and 1973.
In 1969, 71 children from the Saku agricultural district of central Japan where parathion and malathion are used extensively were examined because they were experiencing reduced visual acuity (the vision of 50 percent of these children could not be corrected to 20/20 with lenses), a narrowing of the visual fields, and optic neuritis.5 The signs were first noted in the area in 1965, shortly after insecticides became used on a massive scale. Rates of myopia within the Saku district were found to be higher in area where the concentration of organophosphate pesticides in drinking water was higher.
Compared to 100 control subjects in Tokyo, who were less likely to be exposed to organophosphate pesticides, the Saku children were more likely to have experienced classical organophosphate poisoning symptoms, to have drunk well water, played in a rice field, had a history of definite contact with sprayed pesticides, and lived in a home where pesticides were used. The blood level of organophosphates in the Saku children was highly correlated with myopia and astigmatism (structural defects of the eye or lens which cause blurred images).4
Vision and low dose exposure to organophosphates. An experimental beagle study involving low dose, longterm exposure to a highly toxic organophosphate pesticide (ethylthiometon, two year exposure) and a less toxic organophosphate pesticide (fenitrothion, one year exposure) produced myopia in all exposed dogs.6 Myopia persisted at the end of two years, which was one year after cessation of fenitrothion exposure.
While a dose of 0.15 mg/kg' mevinphos (an organophosphate pesticide) elicited grossly detectable poisoning symptoms in pigeons, less than 0.05 mg/kg caused decreased visual responses to target movement, due to effects on one particular type of neuron (i.e., rotundal neurons).
As the authors note, "All this suggests that exposure to organophosphatepesticides can cause substantial visual dysfunctions over a period of time with little or no warning from the usual peripheral signs that dangerous functional changes are occurring.7
Behavior and low dose organophosphate exposure. Rats exposed to malathion exhibited decreased shock avoidance behavior 60 minutes after injection of a dose (50 mg/kg causing no significant effects on red blood c~ll, plasma, or brain cholinesterase activity. Motor activity was depressed at a lower dose level (25 mg/kg).
"The difference in findings,» the authors write, "illustrates the importance of employing more than one type of task in the assessment of behavioral activity....From these data, it appears that malathion may disrupt rat behavior without producing significant inhibition of either blood or brain [cholinesterase] activity....[lt] is suggested that current human screening procedures designed to monitor malathion toxicity be reviewed for their adequacy in detecting sub-clinical behavioral change."8
Long term Intellectual Impairement and organophosphate exposure. The pigeon and rat studies described above indicate that subtle organophosphate induced behavioral changes might be detectable at lower doses than those eliciting cholidesterase inhibition or other classical signs of organophosphate poisoning. Another study 9 investigated chronic effects of acute organophosphate poisoning (JPR 5~3):27) among one hundred humans who had at one time (an average of nine years earlier) experienced acute poisoning which WQUld have resulted in temporary, reversible cholinesterase inhibition. Two children and one college student were among the poisoned subjects.
Compared to 100 nonpoisoned controls matched for age, sex, level of education, occupational class, socioeconomic status, race, and ethnic background, the poisoned subjects exhibited impairments in intellectual functioning, abstract and flexible thinking, and simple motor skills. The poisoned subjects indicated greater distress and greater perceptions of their own disabilities.
Poisoned and nonpoisoned subjects did not differ in hearing ability, vision, electroencephalograms, or clinical serum and blood chemistry evaluations.
The researchers note that although the major deficits among the poisoned subjects were cognitive, standard clinical neurological examinations do not generally detect impairments of higher level cognitive skills and activities.9
Many studies of organophosphate and carbamate toxicity exist in the literature, but differences exist in the quality of studies and specific compounds and symptoms investigated. Questions remain as to the permanency of effects, the dose at which particular types of damage occur, and the relative effects of high dose acute versus low dose chronic exposures to 'different compounds. What is clear, however, is that behavioral effects that can lead to learning difficulties may follow relatively low dose exposure and that permanent learning difficulties may follow sufficiently high dose exposure.
Organic solvents are à group of liquids made of simple organic (carboncontaining) molecules. They are volatile so that they change, in the presence of air and under normal pressure and temperature conditions, from liquids to gases. Inhalation is therefore a major route of exposure, although absorption through skin is another important route.
' All organic solvents are fat-soluble and neurotoxic, producing effects on the central nervous system at some dose. The brain, having a high fat content and very rich blood supply, concentrates high`levels of solvents quickly.
Short-term exposures at low toxicity may produce headaches, nausea, and nasal and mucous membrane irritation, while long term exposure can result in nonspecific narcotic effects (e.g., talkativeness, motor incoordination) that impair work performance. Specific solvents may cause sleep disturbances, nightmares, insomnia, emotional disorders, epileptic seizures, and encephalopathy (a wasting of brain matter).t
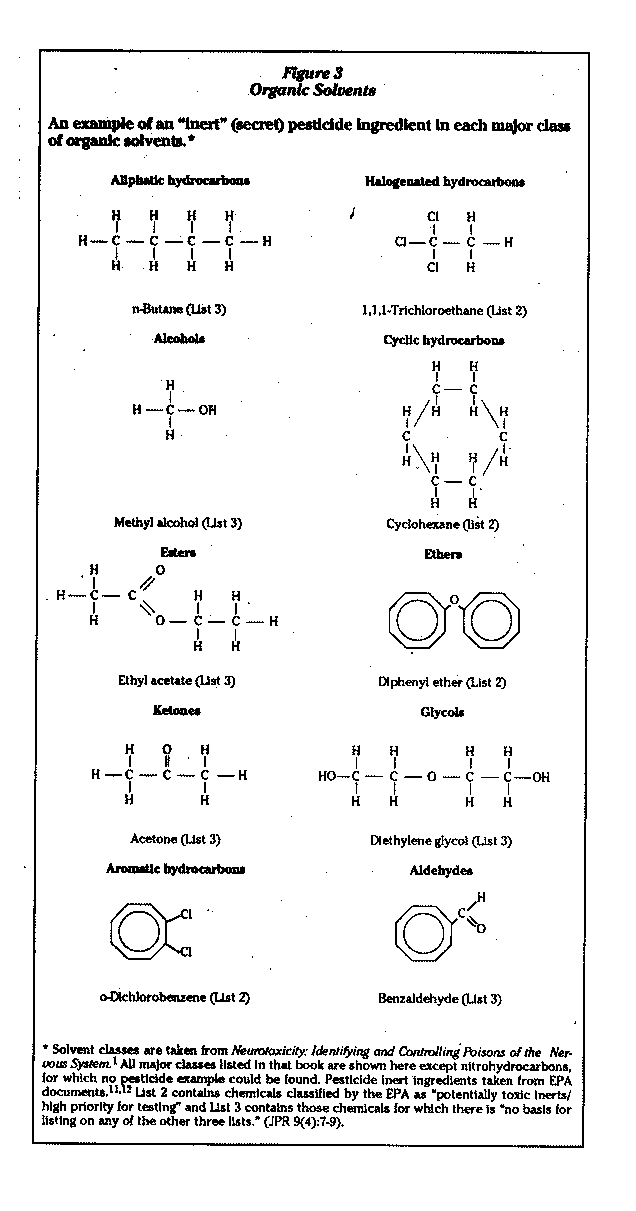
There are a number of major classes of organic solvents; the EPA list of secret Qinert") ingredients allowed for use in pesticide formulations reveals examples of most of the major classes of these solvents (Figure 3).
pesticide ingredients, can damage the inner ear, leading to high-frequency hearing loss. Trichloroethylene, an "inert. pesticide ingredient may damage facial nerves and produce facial numbness. Numbness in hands and feet, muscle weakness, and lack of coordination can be caused by chronic exposure to hexane and methyl-n-butyl ketone, both "inert" pesticide ingredients, and related solvents.
"All organic solvents are fat-soluble and neurotoxic, producing effects on the central nervous system at some dose. The brain, having a high fat content and very rich blood supply, concentrates high levels of solvents quickly."
The effects of long-term low level or short-term exposure to solvents are not well studied. Little effort has been devoted to developing animal models regarding nervous system injury or behavioral disorders in laboratory animals. No neurotoxicity testing of pesticide formulations neurotoxic solvents is required for U.S. pesticide registration.
While workplace exposure to solvents can be reduced by engineering controls, or, less desirably, by personal protection devices, the spraying of pesticides containing unlabeled neurotoxic solvents leaves those who are exposed both uninformed and unprotected. The study of effects on learning or behavior in children who have been exposed to neurotoxic, solvent containing pesticides is hampered by the fact that the presence of the solvents is secret.
Children at a Hawaiian elementary school complained one morning of headaches, stomachaches, breathing difficulties, nausea, and other symptoms. The resultant investigation indicated that the cause of their illness was xylene, the solvent in the pesticide (Dursban 4E) that had been sprayed around the perimeter of the building the day before, not the labeled ingredient, chlorpyrifos.3 This incident involved an acute illness. If subtle, adverse behavioral and learning effects are occurring among certain students following exposure to solvents in pesticides, who would notice or investigate?
Organochlorine compounds, those containing both chlorine and carbon atoms, accumulate in the environment and the human body. Citizens of all industrialized nations carry levels of DDE (a metabolite of DDT), chlordane, heptachlor, PCBs, and other organochlorines in their bodies as a result of exposure to past use of organochlorines as pesticides (e.g., pentachlorophenol, DDT, chlordane, heptachlor, endrin, dieldrin, aldrin, dicofol, toxaphene) and in electrical transformers (PCBs). Dioxins and furans are introduced into humans and their food chains as contaminants in pesticides, pulp and paper products (and the waste products from their manufacture), and municipal incineration. The fungicide pentachlorophenol, for instance, is itself an organochlorine, but is also heavily contaminated with toxic dioxins.
In a human developmental study of the effects of consuming organochlorine-contaminated fish, infants of women who ate an average of two meals per month of fish from the Great Lakes were compared to infants of women who ate less than two Great Lakes fish meals a month. Infants with mothers who ate contaminated fish had smaller birth weight, disproportionately smaller heads, and a shorter gestation period than unexposed infants. At seven months, measurements of these infants' visual recognition-memory were made. Exposed infants were less likely than unexposed infants to recognize and look at a new photograph after having seen one photograph. The most highly exposed infants spent only half the time looking at the new photograph that unexposed infants did. When tested at four years of age, these children exhibited deficits in short term memory on both verbal and quantitative tests.17
The marker organochlorine that was measured in the mothers' umbilical cord, breast milk, and infants was PCB, but the Great Lakes fish would have contained other organochlorines including dioxins) present in the Great Lakes food chain. In fact, two of the fish consumption studies note that newborn behavior deficits were significantly related to mothers' fish consumption, but not PCB levers in the umbilical cord. Two of the researchers hypothesize that "it is possible that those deficits were due to other toxins from the same contaminated fish that were not measured by the analytical laboratory."18
Dioxins and furans are known to be present or potentially present in a large number of pesticides.19 The most toxic dioxin, 2,3,7,8-TCDD, for instance, i5 known to be present in the herbicide dacthal, 20 which massively contaminates the groundwater of eastern Oregon in the onion-growing region of Ontario.21
A study in which mother rhesus monkeys were exposed to 5 parts per trillion (ppt) 2,3,7,8"TCDD in their food for an average of 16 months before giving birth to infants, revealed specific learning difficulties in their Off 2 spring. The young monkeys with exposed mothers exhibited reduced 3 ability (compared to offspring of mothers who were not exposed to dioxin) on a discrimination reversal learning test for shape, but exhibited normal performance on a delayed spatial alternation test. Both these tests are standard behavior tests which measure the time required for 5 monkeys to learn which of several blocks has a reward under it.22 The researchers note that this same effect 6. (i.e., a learning deficit for discrimination reversal learning in the absence of delayed spatial alternation deficits) has been exhibited by monkeys exposed to low levers of lead during development.
This study is particularly disturbing because of the extremely low exposur' levers (i.e., 5 ppt 2,3,7,8"TCDD in the mothers' diet). The U.S. Environmental Protection Agency (EPA) estimates that many Native Americans, Asian Americans, and poor people living along the Columbia River consume large quantifies of fish contaminated with approximately 6.5 ppt 2,3,7,8 TCDD equivalents.23
When the EPA recently calculated risks to people of eating fish contaminated by pulp mill effluents with dioxins and furans, the agency considered only cancer risks and risk of river damage.24 The EPA admitted that reproductive and developmental toxicity is a more sensitive non-cancer effect of dioxin than river damage, but declined to calculate reproductive and developmental risks to humans because not all people consuming dioxincontaminated fish are reproducing and the EPA was only wanting to calculate risks to the "general public." 14.
Despite the lack of required testing, research indicates that certain pesticide ingredients and contaminants can and do cause behavioral and learning deficits. An unknown number of pesticide chemicals and their contaminants are involved in affects on learning. Our children therefore deserve zero exposure to pesticides.

1. U.S. Congress, Office of Technology Assessment. 1990. Neurotoxicity: Identifying and controlling' poisons of the nervous system. OTA-BA-436. Washington, DC: U.S. Government Printing Office.
2.Whyatt, Robin. 1989. Intolerable risk The physiological susceptibility of children to pesticides. 1. Pesticide Reform 9(33:5-9.
3.Young, Bambi Batts. 1986. Neurotoxicity of pesticides . J Pesticide Reform 6(2):8-10.
4.Ishikawa, Satoshi, and Mikio Miyata. 1980. Development of myopie following chronic organophosphate pesticide intoxication: An epidemiological and experimental study. In Merigan, W.H., and B. Weiss (eds.) Neurotoxicity of the visual system. New York Raven Prms.
5.Ishikawa, S. 1970. Eye injury by organic phosphorus insecticide preliminary reports Jap. J. Ophthalmol 15:60-68. Cited in reference 4.
6Suzuki, H. and Ishikawa, S. 1974. Ultrastructure of the ciliary muscle treated by organophosphate pesticides in beagle dogs. Br. J. Ophthalmol. 19:251)-253. Cited in reference 4.
7.Revzein, Alvin. 1980. Effects of organophosphate pesticides and alcohol on visual mechanisms. In Merigan, W.H., and B. Weiss. Neurotoxicity of the visual system. New York. Raven Press.
8. Kurtz, Perry. 1976. Behavioral and bio chemical effects of malathion. Study No. 51-051-73-76. Aberdeen Proving Ground, MD: U.S. Army Environmental Hygiene Agency.
9. Savage, Eidon, Thomas Keefe, Lawrençe Mounce, Robert Heaton, Jamm Lewis, and Patricia Burcar. 1988. Chronic neurological sequelae of acute organophosphate pesticide poisoning. Arch Env Health 43:3845.
10. Dick, RB. 1988. Short duration exposures to organic solvents: The relationship between neurobehavioral test results and other indicators. Neurotoxicology and Teratology 10:39-50. Cited in reference 1.
11. US. Environmental Protection Agency. July 25, 1985. Lists of chemicals used as Inert ingredients in pesticides. Unpublished list.
12. U.S. Environmental Protection Agency. 1989. Inert ingredients in pesticide products; policy statement; revision and modification of liste. Federal Register 54(224):48313-48317 (November 22).
13. Anderson, Bruce (environmental epidemiologist, Hawaii Department of Health). Memorandum to Deputy Director for Environmental Programs re: Waianae Elementary School investigation. Oçtober 20, 1986.
14. U.S. Environmental Protection Agency. 1980. Dioxins. EPA~61)0/2~197. Cited in Van Strum, Carol, and Paul Merrell. 1989. The politics of penta. Seattle, WA: Greenpeace U.S.A.
15. Fein, Creta, Joseph Jacobson,' Sandra Jacobson, Pamela Schwartz, and Jeffrey Kowler. 1984. Prenatal exposure to polychlorinated biphenyls: Effects on birth size and gestational age. J. Pediatr. 105:315~320.
16. Jacobson, S.W., G.G. Fein, J.L. Jacobson P.M. Schwartz. and J.K. Dowler. 1985. The effect of intrauterine PCB exposure on visual recognition memory. Child Dev 56:853-860. Cited in reference 18.
17. Jacobson, Joseph, Sandra Jacobson, and Harold Humphrey. 1990. Effects of in utero exposure to polychlorinated biphenyls and related contaminants on cognitive functioning in young children. J Pediatr 116:3845.
18. Jacobson, Joseph L. and Sandra W. Jacobson. 1988. New methodologies for assessing the effects of prenatal toxic exposure on cognitive functioning in humans. In Marlene Evans (ed.) Toxic contaminants and ecosystem health: A Great Lakes focus. New York John Wiley and Sons.
19. Anonymous. 1985. Pesticides "possibly» contaminated with dioxin list compiled in OPP [U.S. Environmental Protection Agency, Office of Pesticides Programs]. Pesticide and Toxic Chemical News (February 20):34-35.
20. U.S. Environmental Protection Agency. June 6, 1988. DCPA fact sheet. Washington, D.C.
21. Bruck, Clenn. 1986. Pesticide and nitrate contamination of "round water near Ontario, Oregon. Seattle, WA: U.S. Environmental Protection Agency.
22. Schantz, Susan and Robert Bowman. 1989. Learning in monkeys exposed perinatally to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD).Neurotoxicol. and Teratol 11:13-19.
23. McCormack, Craig (Office of Policy Planning and Evaluation, U.S. Environmental Protection Agency), and David Cleverly (Office of Research and Development, U.S. Environmental Protection Agency). April 23, 1990. Analysis of the potential populations at risk from the consumption of freshwater fish caught near paperemills. Draft document. Washington D.C.: U.S. EPA
24. US. Environmental Protection Agency, Office of Water Regulations and Standards. August 1990. Risk assessment for 237 TCDD and 237~TCDF contaminated receiving waters fn~m US chlorinebleaching pulp and paper mills. Washington, D.C.: US. EPA.
Citation for this article: O'Brien, Mary 1990, "Are pesticides taking away the ability of our children to learn?", Journal of Pesticide Reform, Vol. 10, No. 4, Winter 1990 - 1991, pp.4-8.
Copyright © 1990 Northwest Coalition for Alternatives to Pesticides.
Reprinted with permission.
Info Request | Services | Become EAP Member | Site Map
Give us your comments about the EAP site
Ecological Agriculture Projects, McGill University (Macdonald
Campus)
Ste-Anne-de-Bellevue, QC, H9X 3V9 Canada
Telephone:
(514)-398-7771
Fax:
(514)-398-7621
Email: info@eap.mcgill.ca
To report problems or otherwise comment on the structure of this site, send mail to the Webmaster