JPR Index | Virtual Library | Magazine Rack | Search
Sperm counts in healthy men around the world have fallen about 50 percent in the last 50 years. Detailed studies of how sperm counts have changes over time in a particular area show the same pattern, with a few exceptions. Researchers hypothesize that exposure to toxic chemicals may be an important cause of the decline.
In laboratory tests, researchers exposed pregnant or nursing mother rats to certain chemicals found in pesticide products. This exposure disrupted the hormonal balance in their male offspring and limited the development of their sperm~producing cells, resulting in permanently reduced sperm courts.
Over 50 pesticides are known to disrupt sperm production or male hormones. Some of these pesticides are among the most commonly used pesticides in the U.S. in both agricultural and household situations. About 200 million pounds of sperm damaging pesticides are used in agriculture every year, and over half a billion applications of these same pesticides are made in our homes and gardens.
Chemicals that can have so dramatic an effect on our physiology do not belong on our farms, in our communities, or in our homes.
BY CAROLINE Cox
No New Dads in the Plant," screams the headline. "The men noticed it first, swapping stories over lunch," continues the article. "None had fathered children lately." The year was 1977, and the men manufactured a pesticide commonly known as DBCP in the central California town of Lathrop. "I started looking around and there weren't any children being bore," said a union steward. So begins one of the first chapters in the story of how pesticides impact male fertility.
Since 1977, the story has grown. Not only have scientists collected evidence that human sperm production has decided over the lest half century, but the list of pesticides known to disrupt sperm production or male hormones continues to lengthen.
In 1992, when four Danish scientists published a study suggesting that sperm counts in men worldwide had declined about 50 percent since 1940,2 the story made headline news. Sperm are a man's immediate and personal connection to the future of our species, and the disappearance of half of this connection is hard to ignore. "Every man in this room," a wildlife biologist told a hearing before a subcommittee of the U.S. House of Representatives, "is half the man his grandfather was."3 His audience listened.
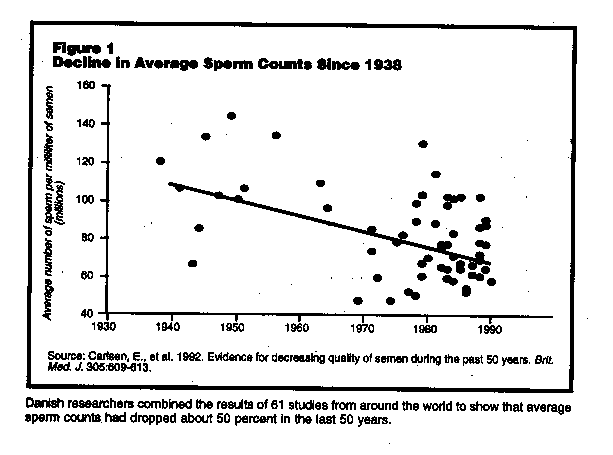
This study, the first widely publicized study of trends in human sperm counts in the lest half-century, was authored by research fellow Elisabeth Carlsen and a team of Danish scientists.2 Carlsen and her colleagues analyzed the results of over 60 studies of sperm counts published between 1938 and 1991 with what they called a "meta-analysis," a statistical analysis that linked results of a large number of independent studies. Using a model which assumed that sperm counts changes over time in a linear way, the results of the meta-analysis indicated average sperm counts declined from 113 million per milliliter (ml) of semen to 66 million per ml during the half century for which they had data (See Figure 1.) The studies came from around the world, with about half from in the U.S. The results had truly profound implications were such a decline to continue, the human race would be unable to reproduce beginning sometime in the next century.
Since Carlsen's study was published, three other studies have found similar declines in sperm counts in smaller groups of men! Researchers at the University Hospital in Ghent, Belgium, found that counts among their sperm donors had declined about 10 million per ml between 1977 and 1994.4 At Edinburgh, Scotland's Centre for Reproductive Biology, Stewart Irvine found that median sperm counts among its sperm donors had declined about 40 percent when he compared men born in the 1940s with men born in the late 1960s.5 At a sperm bank in Paris, France, mean sperm counts among donors declined by about 2 percent per year from 1973 to 1992, for a total decline of 32 percent.6
Older studies show a similar pattern: sperm counts in Washington D.C. dropped about 25 percent during the 1980s7 and sperm counts in Denmark dropped about 25 percent between 1952 and 1972.5
Perhaps of greater concern, these studies found that other measures of sperm quality also showed problems; both the amount of semen produced and the vigor of the sperm declined. Carlsen's study found that semen volume decreased about 20 percent. In addition, the proportion of men with sperm counts below 20 million per ml (sperm counts this low are referred to as "subfertile" 2) tripled. The Belgian study found chat both the proportion of abnormal sperm and their mobility decreased during the lest 20 years.4 The French study had similar, and just as unsetling, results.6

Particularly telling were comments made by Dr. Pierre Jouannet, one of the scientists involved in the French study. "We always had the idea chat there was no decline in sperm characteristics,»9 he explained. In fact, he and his colleagues began the study because they believed it would overturn Carlsen's hypothesis of a "general decline in the quality and quantity of sperm,--at least, in Paris."9 The results, showing just the opposite, astonished everyone involved.
Again, older studies show similar results. The Danish study mentioned above found that between 1952 and 1972 the proportion of abnormal sperm increased (from 26 percent to 45 percent) and sperm movement decreased.8 In Oslo, Norway, the proportion of abnormal sperm rose from 40 percent to 59 percent between 1966 and 1986.
Further evidence of a large-scale problem come. from studies of other male reproductive disorders. The incidence of testicular cancer has increased as much as 3 or 4 times since the 1940s. The incidence of undescended testes and other anatomical abnormalities of male genitals also seems to have increased."
These results, not surprisingly, have not been accepted uncritically. Several researchers felt Carlsen's results could be a statistical artifact, or caused by changes in sperm counting equipment. A team of researchers, most of whom were employed by Dow Chemical Company, pointed out chat the data used by Carlsen and her coworkers could be analyzed with different statistical models The three models that seemed to fit the data best showed a 50 percent decline around 1965, but a constant or slighty increasing sperm count in the years since 1970.
In addition, a recent analysis of sperm counts from three U.S. cities (New York, New York; Roseville, Minnesota; and Los Angeles, California) indicated that sperm counts in those cities had not declined in the lest twenty-five years. A study from southern France found no changes between 1977 and 1992. A study of Seattle-area college students found similar results
Sperm counts vary enormously between countries or regions, between individual men, and even between counts on the same men. Therefore it is not surprising that not all analyses of sperm counts find the same patterns. Whether the decline in sperm counts observed by Carlsen and others is in fact world wide, or whether it includes only certain geographical areas, the overall conclusion is clear: we should act now to protect our reproductive health.
Studies of sperm counts over time leave a critical question unanswered. What could account for a precipitous decline in sperm production by otherwise healthy men? Carlsen suggested that environmental causes were likely, particularly those toxins that could affect human hormone systems.2 Richard Sharpe, a research physiologist in Edinburgh, Scotland, developed a more specific hypothesis, and suggested that the decline "is the result of endocrine changes in fetal/prepubertal life [prior to birth or during childhood]."20
This hypothesis paints a particularly chilling picture. The endocrine system is made up of the glands and hormones (chemical messengers) that regulate growth, development, behavior, and sexuality. Sharpe hypothesized that this complex system might be disrupted before birth or during childhood by substances acting like natural hormones. The result is a permanent impairment of the reproductive system.
In particular, he hypothesized that hormone disruption at a sensitive time in development could block the development of Sertoli cells, cells within the testes that "nurse" sperm cells as they develop. The number of Sertoli cells sets a cap on the number of sperm which a man is able to produce; therefore a chemical exposure that blocked hormone involved with Sertoli cell development would irreversibly limit sperm production.
The hormones Sharpe thought might be important in determining adult sperm production are follicle-stimulating hormone (FSH) and estrogens. Like most hormones, these have multiple functions in our bodies. Their relevance to sperm production is that FSH in juvenile mammals promotes multiplication of the Sertoli cells. Without enough FSH, fewer Sertoli cells are produced. Levels of FSH are regulated by estrogens; higher levels of estrogen result in lower levels of FSH. So Sharpe hypothesized that synthetic chemicals acting like estrogens might lower levels of FSH, resulting in fewer Sertoli cells and permanently decreased sperm production.
A laboratory test of this hypothesis has been completed. Sharpe and his colleagues studied mother rats who drank water contaminated with two synthetic chemicals, octyl phenol and butyl benzyl phthalate that are known to act like estrogens. The rats used in the study were pregnant and nursing; the study spanned the interval when their male offspring would be developing Sertoli cells. The results fit Sharpe's hypothesis perfectly sperm production was reduced (10 to 20 percent) in the offspring of the rats drinking contaminated water and the number of Sertoli cells (as estimated by testes size) was reduced.
The development and growth of the male reproductive system is obviously a complex process. It is therefore not surprising that synthetic chemicals might effect male fertility in more than one way. Earl Gray, a toxicologist with the U.S. Environmental Protection Agency, studied how dioxin '(2,3,7,8-tetrachlorodibenzo-p-dioxin) exposure of mothers affects sperm production in their male offspring.22 In both rats and hamsters, a single small exposure (1-2 micrograms per kilogram of body weight) during a sensitive stage of pregnancy resulted in permanent decreases of up to 60 percent in the sperm count of male offspring. Dioxin likely causes this decrease in a completely different manner than the mechanism demonstrated by Sharpe; it appears to affect growth factors rather than involving estrogens.
All three of the chemicals discussed above are found in pesticide products. Octyl phenol and butyl benzyl phthalate are both used as "inert" ingredients, ingredients used in a pesticide product to make it more efficient or easier to use. Dioxin is a contaminant of at least one currently-used pesticide, the herbicide 2,4-D. This connection leads to several other questions. Are there other pesticides that adversely affect sperm? Has the use of pesticides contributed to the decline in sperm counts? Three different kinds of evidence point to pesticides as part of the problem facing men today:
· Several organochlorine pesticides have had dramatic impacts on male fertility. In 1975, a worker from a chemical factory in Hopewell, Virginia visited his family physician for help with persistent headaches, tremors and irritability. Further investigations showed that he, and his fellow workers, were contaminated with chlordecone, an insecticide made at the Hopewell factory, and that only one quarter of the workers at the plant had normal sperm counts. The sperm produced by these workers also did not swim as well as normal sperm. The workers' sperm counts increased over the next five years as medications removed chlordecone from their body tissues.23
Dibromochloropropane (DBCP), a soil fumigant, became notorious in the late 1970s because of its ability to reduce or eliminate sperm production in exposed workers. A 17-year follow-up study of 15 exposed workers found that recovery had occurred in only 6 of them.24 Workers who were able to father children had mostly girls; less than 20 percent of the children born to men with the lowest sperm counts were sons.24 In laboratory tests, exposure of pregnant rats to DBCP caused small and abnormal testes in their male offspring.25
The story of how a pesticide as toxic as DBCP became widely used, both in the U.S. and abroad, is basically a story of corporate greed. The first toxicology tests on DBCP were clone in 1954 and 1955. Even at the lowest doses tested, DBCP caused damage to testes. Shell and Dow Chemical Companies, manufacturers of DBCP, estimated a "safe" exposure lever for workers exposed to DBCP, but it was not based on any actual data. When the researchers who had clone the toxicology tests produced a product data summary to g~ve customers, Shell advised them to understate hazards and exclude some toxic effects. The manufacturer convinced the U.S. Department of Agriculture to require only mild safety warnings on the labels of DBCP products.26
· Over 50 currently used pesticides have caused problems related to male fertility in laboratory or clinical tests. Some of these pesticides are among the most commonly used pesticides in the U.S. (See Table 1 for a complete list.) Eight out of the 25 pesticides most extensively used in U.S. agriculture 27 have adversely affected sperm production or the functioning of sex hormones in laboratory animals or humans. Estimated annual use of these chemicals totals nearly 200 million pounds, about 25 percent of total agricultural pesticide use. Seven out of the top ten pesticides used in commercial and industrial situations 27 have similar effects; their use accounts for almost 80 percent of this kind of pesticide use. Similar effects have also been shown by 8 out of the 25 pesticides most commonly used in American households.28 We make an astonishing five hundred million applications of these chemicals in our homes every year.
· Pesticide exposure is associated with infertility. Large-scale studies assessing pesticide exposure and its relationship to infertility have not been done. However, several small studies have demonstrated this relationship. Patients treated at Vienna, Austria's Institute of Sterility Treatment, because they produced little sperm or sperm of low quality, were ten times as likely to work in agriculture as those referred to the clinic for other reasons.29 In the Netherlands, wives of farmers who applied pesticides took longer to become pregnant, and became pregnant less often, than wives of farmers with less pesticide exposure.30
Precaution and prevention must be our watchwords as we respond to the new research regarding declines in sperm production. Sperm are "canaries in the coal mine" that help us begin to understand the many effects that pesticides can have on our health and the health of the wildlife around us. Take the information in this article to the people who make pesticide use decisions in your community, in your state, and in our country. Talk to your school board, your city councils, your county commissioners, your state legislators, your representative, or your senator. Tell them that for your own health and the health of future generations of both people and wildlife we need to promote alternatives to sperm damaging pesticides as aggressively as the chemicals themselves have been promoted. Tell them that chemicals having so dramatic an effect on our physiology do not belong in our communities. After all, it's our future.
1. No new dads in the plant. Farm chemical suspended.' Grants Pass Daily Courier. August 5,1977. 3.
2. Carlsen, E., et al. 1992. Evidence for decreasing quality of semen during the past 50 years. Brit Med. J. 305:609-613.
3. Pinchbeck, D. 1996. Downward motility. Esquire (January):80-84.
4. Van Waeleghem, K. et al. 1994. Deterioration of sperm quality in young Belgian men during recent decades. Human Repro. 9 (Suppl. 4): 73.
5. Irvine, D.S. 1994. Falling sperm quality. Brit Med 1 309:476.
6. Auger, J. et al. 1995. Decline in semen quality among fertile men in Paris during the past 20 years. M Engl J. Mad.: 332:281-285.
7. Leto, S. and F.J. Frensilli. 1981. Changing parameters of donor semen. Fen Steril. 36(6): 766-770.
8. Bostoffe, E., J. Serup, and H. Reba. 1983. Has the fertility of Danish men declined through the years in terms of semen quality? A comparison of semen qualifies between 1952 and 1972. Nit J. Fertil. 28(2):91-95.
9. Wright, L.1996. Silent sperm,. (A reporter at large.) The New Yorker (Jan. 15):42,48,50-53,55.
10. Bendvold, E.1989. Semen quality in Norwegian men over a 20-year period. Int. J. Fertil. 34(6):401~104.
11. Carlsen, E. et al. 1995. Declining semen quality and inc~easing incidence of testicular cancer: Is there a common cause. Environ. Health Persp. 103 (Suppl. 7):137-139.
12. Bromwich, P. et al.1994. Decline in sperm courts: an artifact of changes reference range of normal'? Brit Med. J. 309:19-22.
13.Tummon, I.S. and D. Mortimer. 1992. Decreasing quality of semen. Brit Med J.305:1228-1229.
14. Brake, A. and Krause, W.1992. Decreasing quality of semen. Brit Med. J. 305:1498.
15. Olsen, G.W. et al.1995. Have sperm counts been reduced 50 percent in 50 years? A statistical model revisited. Fert. Steril 63(4):887-893.
16.Fisch, H. et al.1996. Semen analyses in 1,283 men from the United States over a 25-year period: No decline in quality. Fert. Steril 65(5):1009-1014.
17. Bujan, L. et al.1996. Time series analysis of sperm concentration in fertile men in Toulouse, France between 1977 and 1992. Brit. Med. J. 312:471472.
18. Paulsen, C.A. et al. 1996. Data from men in Greater Seattle area reveals no downward trend in semen quality: Further evidence that deterioration of semen quality is not geographically uniform. Fen Steril 65(5):1015-1020.
19. Sharpe, R.M.1993. Commentary: Declining sperm counts in men--is there an endocrine cause?' J. Endocrinol 136:357-360.
20. Sharpe, R.M. and N.E. Skakkebask. 1993. Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract? The Lancet 341 :1392-1395.
21. Sharpe, R.M. et al. 1995. Gestational and lactational exposure of rats to xenoestrogens results in reduced testicular size and sperm production. Environ. Health Persp. 103(12):1136-1143.
22. Gray, L.E. et al. 1995. Exposure to TCDD during development permanently alters reproductive function in male Long Evans rats and hamsters: Reduced ejaculated and epididymal sperm numbers and sex accessory gland weights in offspring with normal androgenic statue. Toxicol Appl Phammacol 131:108-118.
23. Guzelian, P.S. 1982. Comparative toxicology of chlordecone (Kepone) in humans and experimental animals. Ann Reu Phammacol Toxicol 22:89-113.
24. Potashnik, G. and A. Porath. 1995. Dibromochloropropane (DBCP): A 17-year reassessment of testicular function and reproductive performance. l Occup. Environ Med 37(11):1287-1292.
25. Warren, D.W., N. Ahmad, and P.K. Rudeen.1988. The effects of fetal exposure to 1,2-dibromo-3chloropropane on adult male reproductive function. Biol Repro. 39:707-716.
26. Thrupp, L.A. 1991. Sterilization of workers from pesticide exposure: The causes and consequences of DBCP-induced damage in Costa Rica and beyond. Inter. J. Health Serv.21(4):731-757.
27. Aspelin, A.L. 1994. Pesticides industry sales and usage: 1992 and 1993 market estimates. Washington, D.C.: U.S. EPA. Office of Pesticide Programs. Biological and Economic Analysis Division.
28. Whitmore, R.W., J.E. Kelly, and P.L. Reading. 1992. National home and garden pesticide use survey. Final report, vol. 1: Executive summary results, and recommendations. Research Triangle Park, NC: Research Triangle Institute.
29. de Cock, J. et al. 1994. Time to pregnancy and occupational exposure to pesticides in fruit growers in The Netherlands. Occup. Environ. Med. 51 :693-699.
30. Strohmer, H. et al. 1993. Agricultural work and infertility. Am. J. Ind Med 24:587-592.

1. Behera, B.C. and S.P. Bhunya. 1989. Studies on the genotoxicity of asataf (acephate). an 21 organophosphate insecticide, in a mammalian in vivo system. Mut Res. 223-287-293.
2. Lutz, H. and Lutz-Ostertag, Y. 1972. The action of different pesticides on the development 22 of bird embryos. In Klingberg, M.A. et al (eds.). Drugs and fetal development (Advances in Experimental Medicine and Biology. Vol. 27). 23 New York, NY: Plenum Press.
3. Wyrobeck, A.J. et al. 1981. Sperm shape abnormalities in carbaryl-exposed employees. Env. Health Persp. 40:255-265.
4. Shtenberg, A.l. and M.N. Rybakova. 1968. Effect of carbaryl on the neuroendocrine system of rats. Fd Cosmet Toxicol 6:461-467.
5. Yousef, M.l. 1995. Toxic effects of carbofuran and glyphosate on semen characteristics in 25 rabbits. J. Environ Sci Health B30:513-5343.
6. Pant, N. et al. 1995. Effect of oral administration of carbofuran on male reproductive sys- 26 tem of rat. Hum. Exp. Toxicol 14:889994.
7. Mikhail, T.H. et al. 1979. Acute toxicity of organophosphonus and-organochlorine insecticides in laboratory animals. Z Emahnungswiss 27 1 8:258-268.
8. Sherman, J.D. 1995. Chlorpyrifos (Dursban)-associated birth defects: A proposed syndrome, report of four cases, and discussion of the toxicology. Intem. 1 Occup. Med. Toxicol 4:417431.
9. U.S. Dept. of Health and Human Services. Public Health Service. Agency for Toxic Substances and Disease Registry. 1994. Toxicological profile for diazinon (Draft.) (August.) p.41.
10. Salem, M.H. et al. 1988. Effect of organophosphorus (dimethoate) and pyrethroid (deltamethrin) pesticides on semen characteristics in rabbits. l Envir Sci. Health B23:279-290.
11. U.S. Dept. of Health and Human Services. Public Health Service. Agency for Toxic Substances and Disease Registry. 1995. Toxicological profile for disulfaton (August.) p.65.
12. Saxena, P.K. and K. Mani. 1985. Quantitative study of testicular recrudescence in the fresh water teleost, Channa punctatus (Bl.) exposed to pesticides. Bull Environ. Conl Toxicol 34:597-607.
13. Balasubramanian, K. et al. 1987. Effect of malathion on the testis of male albino rats. Med. Sci Res. 15:229-230.
14. Mathew, G., K.K. Vijayalaxmi, and M.A. Rahiman. 1992. Methyl parathion-induced 34 sperm shape abnormalities in mouse. Mut Res.280:169-173.
15. Hemavathy, K.C. and N.B. Krishnamurly. 1987. Evaluation of Lannate 20, a carbamate insecticide in the germ cells of male mice. Environ. Res. 42:362-365.
16. Schein, L.G. et al. 1980. Effects of pesticides on 3H-dihydrotestosterone binding to cytosol proteins from various tissues of the mouse. J. Environ. Pathol Toxicol 3:461-470.
17. Chou, K.C. and R.M. Cook. 1994. Paraoxon inhibits fertilization of mouse gametes in vitro. Bull Envinron. Contam. Toxicol 53:863-868.
18. Saxena, A.K. and K. Sarin. 1980. Histopathological and biochemical changes in the river and testes of desert gerbil, after repeated exposures of Thimet (phorate). Toxicology 18:133-144.
19. U.S. EPA. OPTS. 1987. Guidance for the reregistration of pesticide products containing phosphamidon as the active ingredient. Washington, D.C. (Dec.). p.15.
20. Behera, B.C. and S.P. Bunya. 1987. Genotoxic potential of an organophosphate insecticide phosphamidon (dimecron): an in vivo study in mice. Toxicol Lett. 37:269-277.
21. El Nahas, S.M., H.A. de Hondt, and H.E. Abdou. 1989. Chromosome aberrations in spemmatogonia and sperm abnommalities in Curacron-treated mice. MutL Res. 222: 409-414.
22. Kumari, J. and N.B. Kfishnamurly. 1986. Mutagenicity studies with safrotin in Drosophila melanogaster and mice. Environ. Res. 41:44-52.
23. Bunya, S.P. and P.C. Pati 1988. Genotoxic effects of a synthetic pyrethroid insecticide cypermethrin, in mice in vivo Toxicol Lett. 41 :223-230.
24. Bhunya, S.P. and P.C. Pati. 1990. Effect of deltamethrin, a synthetic pyrethroid, on the induction of chromosome aberrations, micronuclei and sperm abnommalities in mice. Mutagenesis 5:229-232.
25. Pati, P.C. and S.P. Bunya. 1989. Cytogenetic effects of fenvalerate in mammalian in vivo test system. Mut. Res. 222:149-154.
26. Eil, C. and B.C. Nisula. 1990. The binding properties of pyrethroids to human skin fibroblast androgen receptors and to sex hormone binding globulin. J. Steroid Biochem. 35:409-414.
27. U.S. EPA. Office of Pesticide Programs. Health Effects Division.1995. Use of chemicals evaluated for carcinogenic potential. Memo from Stephanie Irene, acting director, to Health Effects Division branch chiefs, et al, Washington, D.C: (Aug. 7.)
28. Singh, S.K. and R.S. Pandey. 1989. Gonadal toxicity of short term chronic endosulfan exposure to male rats. Ind J. Exper. Biol:27:341-346.
29. Pandey, N. et al.1990. Studies on the genotoxicity of endosulfan, an organochlofine insecticide, in mammalian germ cells. Mut Res. 242:1-7.
30.Dikshith, T.S.S. and K.K. Dana. 1972. Effect of intratesticular injection of lindane and endrin on the testes of rag. Acta Pharmacol. Toxicol. 31:1-10.
31. Chowdhury, A.R., H. Venkatakfishna-Blan, and A.K. Gautam. 1987. Testicular changes of rats under lindane treatment. Bull Environ Contam. Toxicol 38:154-156.
32. Gray, L.E. et al. 1989. A dose-response analysis of methoxychlor-induced alterations of reproductive development and function in the rat. Fund Appl Toxicol 12:92-108.
33. vom Saal, F.S. et al.1995. Estrogenic pesticides: .so binding relative to estradiol in MCF-7 cells and effects of exposure during fetal life on subsequent territorial behavior in male mice. Toxicol Lett. 77:343-350.
34. Salama, S.A., A.l. Fahmi, and G.E.S. Abo ElGhar. 1995. Chromosomal aberrations and spermhead abnommalities induced by abamectin 54. (avemmectin B1) and its degradates in male Swiss albino mice. Cytologia 60:411-417.
35. Fail, P.A. et al. 1991. Reproductive toxicity of bofic acid in Swiss (CD-1) mice: Assessment using the continuous breeding protocol. Fund Appl Toxicol 17:225-239.
36. Barnes, T.B., A.J. Verlangieri, and M.C. Wilson. 1983. Reproductive toxicity of methyl-1-(butylcarbamoyl)- 2-benzimidazole carbamate (benomyl) in male Wistar rats. Toxicology 28:103-115.
37. Piersma, A.H., A. Verhoef, and P.M. Dortant. 1995. Evaluation of the OECD 421 reproductive toxicity screening tests protocol using 1(butylcarbamoyl) - 2-benzimidazolecarbamate (Benomyl). Teratog. Carcinog. Mutag.15:93-100.
38. Gray, L.E. et al. 1990. Carbendazim-induced alterations of reproductive development and function in the rat and hamster. Fund Appl Toxicol 15:281 -297.
39. Carter, S.D., R.A. Hess, and J.W. Laskey. 1987. The fungicide methyl 2-benzimidazole carbamate causes infertility in male Sprague-Dawley rats. BioL Repro. 37:709-717.
40. Shivanandappa, M.K. Kfishnakumari, and S.K. Majumder. 1983. Testicular atrophy in Gallus domesticus fed acute doses of copper fungicides. Poul,' Sci 62:405-408.
41. Quinto, l. et al. 1989. Effect of DNOC, ferbam and imidan exposure on mouse sperm morphology. Mut Res. 224:405-408.
42. Hemavathi, E. and M.A. Rahiman. 1993. Toxicological effects of ziram, thiram, and Dithane M-45 assessed by sperm shape abnommalities in mice. l Toxicol Exp. Health 38:393-398.
43. Gray, L.E., J.S. Ostby, and W.R. Kelce. 1994. Developmental effects of an environmental antiandrogen: The fungicide vinclozolin alters sex differentiation of the male rat. Toxicol Appl Phammacol 129:46-52.
44. U.S. EPA. OPPTS. 1995. Reregistration eligibility decision (RED): Asulam. Washington, D.C. (Sept.) p.15.
45. Kniewald, J. et al. 1995. Effect of s-triazine compounds on testosterone metabolism in the rat prostate. l Appl Toxicol 15:215-218.
46. Ivanova-Chemishanka, L. and G. Antov. 1980. Changes in the gonads reproduction and generations of white rats under the effect of some pesticides. Arch Toxicol (Suppl. 4):459-462.
47. Lerda, D. and R. Rizzi. 1991. Study of reproductive function in persons occupationally exposed to 2,4-dichlorophenoxyacetic acid (2,4D). Mut Res. 262:47-50.
48. Seiler, J.P. 1979. Phenoxyacids as inhibitors of testicular DNA synthesis in male mice. Bull Environ. Conl Toxicol 21 :89-92.
49. U.S. EPA. ORD. 1994. Estimating exposure to dioxin-like compounds. Vol. II. Properties, sources, occurrence and background exposures. Washington, D.C. (June.) p.3-58.
50. Gray, L.E. et al.1995. Exposure to TCDD during development permanently alters reproductive function in male Long Evans rag and hamsters. Toxicol Appl Phammacol 131:108-118.
51. National Toxicology Program. 1992. NTP technical report on toxicity studies of glyphosate administered in dosed feed to F344/N rats and B6C3F1 mice. (92-3135). National Institutes of Health. (July.)
52. U.S. EPA. Office of Prevention! Pesticides and Toxic Substances. 1995. Reregistration eligibility decision (RED): Linuron. Washington, D.C. (March.) p.13.
53. Rios, A.C.C. et al. 1995. The action of the pesticide paraquat on somatic and germ cells of mice. Mut Res. 328:113-118.
54. U.S. EPA. Office of Prevention, Pesticides and Toxic Substances. 1994. Reregistration eligibility decision(RED): Pronamide. Washington, D.C. (May.) p.11.
55. Dshurov. A. 1979. Histological changes in organs of sheep in chronic simazine poisoning. [German with English abstract.] Zbl Vet Med. A26:44-54.
56. U.S. EPA. Office of Pesticides and Toxic Substances. 1983. Memo from A. Arce to R. Taylor. (Oct. 13.)
57. U.S. EPA. Office of Pesticides and Toxic Substances. 1981. Memo from W. Dykstra to R. Taylor. (Oct. 26.)
58. U.S. Dept of Health and Human Services. Public Health Service.,Agency for Toxic Substances and Disease Registry. 1993. Toxicological profile for chromium. (April.) p.57.
59. Eustis, S.L. et al.1988. Toxicology and pathology of methyl bromide in F344 rats and B6C3F1 mice following repeated inhalation exposure. Fund Appl Toxicol 11: 594-610.
Citation : Cox, Caroline. 1996. "Masculinity at risk", Vol. 16, No. 2, pp 2-7
Copyright © 1996 Northwest Coalition for Alternatives to Pesticides.
Reprinted with permission.
Info Request | Services | Become EAP Member | Site Map
Give us your comments about the EAP site
Ecological Agriculture Projects, McGill University (Macdonald
Campus)
Ste-Anne-de-Bellevue, QC, H9X 3V9 Canada
Telephone:
(514)-398-7771
Fax:
(514)-398-7621
Email: info@eap.mcgill.ca
To report problems or otherwise comment on the structure of this site, send mail to the Webmaster